plantprowler
Chemical
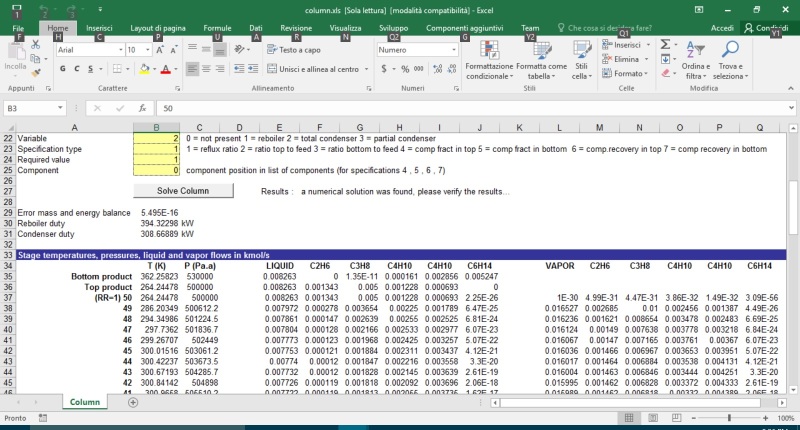
If the specifications on a certain lower boiling impurity in the product is only 0.5 ppm is it feasible to reach such tight specs using distillation as a separation procedure?
Or is distillation typically not the right seperation-operation choice for achieving such tight ppm level specs in products. The two substances are quite far apart in normal Boiling Points. 150 C (impurity) vs 220 C (main product). Actual distillation is under high vacuum so the BPs may be closer together at 10 mm Hg. In any case, Antoine equations are available for both.
Do the standard Distillation Design procedures (e.g McCabe Thiele or more refined variations ) hold for designing and calculating the right number of stages?
Or is distillation typically not the right seperation-operation choice for achieving such tight ppm level specs in products. The two substances are quite far apart in normal Boiling Points. 150 C (impurity) vs 220 C (main product). Actual distillation is under high vacuum so the BPs may be closer together at 10 mm Hg. In any case, Antoine equations are available for both.
Do the standard Distillation Design procedures (e.g McCabe Thiele or more refined variations ) hold for designing and calculating the right number of stages?