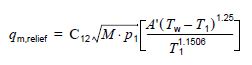Hasab
Chemical
- Oct 7, 2019
- 28
Hello my friends ![[bigsmile] [bigsmile] [bigsmile]](/data/assets/smilies/bigsmile.gif) ,
,
I am dealing with over-pressure scenario where a PSV is set for exchanger blocked in on the cold side (the cold side contain only vapor fluid) with flow in the hot side, as i know there is an explanation for this scenario in API 521 where the fluid in the cold side is liquid not vapor (Hydraulic Expansion), which the suggestion there is a nominal diameter of (DN) 20 × DN 25 (NPS 3/4 × NPS 1) relief valve is commonly used. but if the fluid in the cold side is gas instead of liquid, is there any rule or equation explain how to determine the required flow rate through the PSV in this instance?
( As per my understanding, the over-pressure in this case will result form the expansion of the trapped gas (might be in the tube or the shell) heated up by the hot side fluid, i did not find any standard or equation can be used to determine the required flow rate for this contingency).
could someone have idea give any help or suggestion. thanks to everyone in advance.
![[bigsmile] [bigsmile] [bigsmile]](/data/assets/smilies/bigsmile.gif) ,
,I am dealing with over-pressure scenario where a PSV is set for exchanger blocked in on the cold side (the cold side contain only vapor fluid) with flow in the hot side, as i know there is an explanation for this scenario in API 521 where the fluid in the cold side is liquid not vapor (Hydraulic Expansion), which the suggestion there is a nominal diameter of (DN) 20 × DN 25 (NPS 3/4 × NPS 1) relief valve is commonly used. but if the fluid in the cold side is gas instead of liquid, is there any rule or equation explain how to determine the required flow rate through the PSV in this instance?
( As per my understanding, the over-pressure in this case will result form the expansion of the trapped gas (might be in the tube or the shell) heated up by the hot side fluid, i did not find any standard or equation can be used to determine the required flow rate for this contingency).
could someone have idea give any help or suggestion. thanks to everyone in advance.