hirschaplin
Petroleum
Hello,
Typically I use HYSYS or ChemCAD to find out the condensation temperature of my gas mixture at a certain pressure. Information that I need to know this when working with compressors and trying to understand the importance of a KO drum and how to design it.
However, I have been building up a sweet little excel sheet that is tailored with all kind of calculations that I need for my work... It would be very helpful to also calculate the condensation temperature in my excel sheet to avoid the extra step of setting up the process in HYSYS or ChemCAD just to get the condensation temperature.
I have already built a simple gas mixing feature in excel based on a gas database including most of the typical properties for each gas that I can use when mixing my gas.
Example stream at 10 deg C and 1,61 bar abs. with the following gas composition:
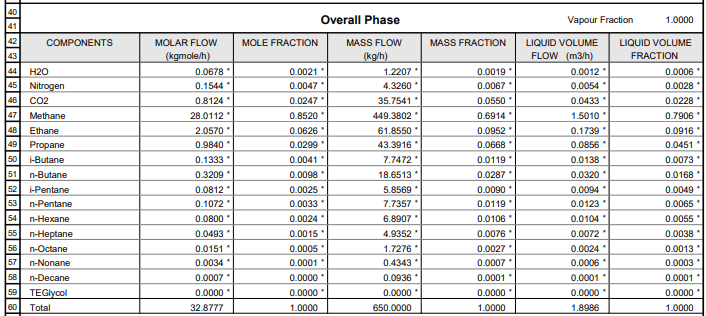
HYSYS tells me that the condensation temperature for this stream is -6.466 deg C. How can I calculate the same result within +/- 10% in my excel sheet?
Typically I use HYSYS or ChemCAD to find out the condensation temperature of my gas mixture at a certain pressure. Information that I need to know this when working with compressors and trying to understand the importance of a KO drum and how to design it.
However, I have been building up a sweet little excel sheet that is tailored with all kind of calculations that I need for my work... It would be very helpful to also calculate the condensation temperature in my excel sheet to avoid the extra step of setting up the process in HYSYS or ChemCAD just to get the condensation temperature.
I have already built a simple gas mixing feature in excel based on a gas database including most of the typical properties for each gas that I can use when mixing my gas.
Example stream at 10 deg C and 1,61 bar abs. with the following gas composition:

HYSYS tells me that the condensation temperature for this stream is -6.466 deg C. How can I calculate the same result within +/- 10% in my excel sheet?

![[thumbsup2] [thumbsup2] [thumbsup2]](/data/assets/smilies/thumbsup2.gif)