JohnWeal
Mechanical
- Dec 16, 2012
- 124
Good Afternoon,
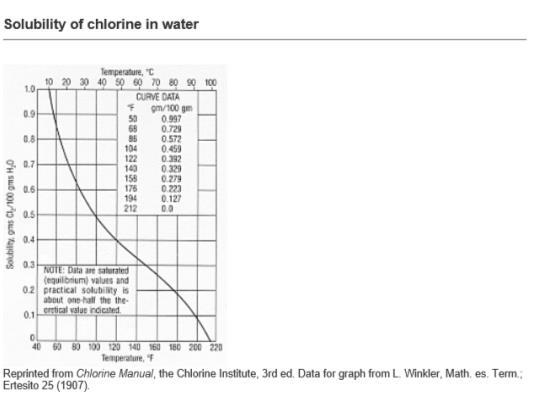
I have read in some guidance notes that for a chlorine in water solution on cooling below 9.6 deg C, crystals of Chlorine Hydrate are deposited.
We may be dosing a solution of 3 ppm, 5 ppm or 7 ppm and the water treatment facility is located where the temperatures are from -10 deg C to +50 deg C.
If the pipework lagging / insulation is only good for keeping the solution at minimum 5 deg C for a -10 deg C ambient temp, how much chlorine hydrate is likely to form?
Regards
John
I have read in some guidance notes that for a chlorine in water solution on cooling below 9.6 deg C, crystals of Chlorine Hydrate are deposited.
We may be dosing a solution of 3 ppm, 5 ppm or 7 ppm and the water treatment facility is located where the temperatures are from -10 deg C to +50 deg C.
If the pipework lagging / insulation is only good for keeping the solution at minimum 5 deg C for a -10 deg C ambient temp, how much chlorine hydrate is likely to form?
Regards
John