The process of Cl2 liquefaction is detailed here:
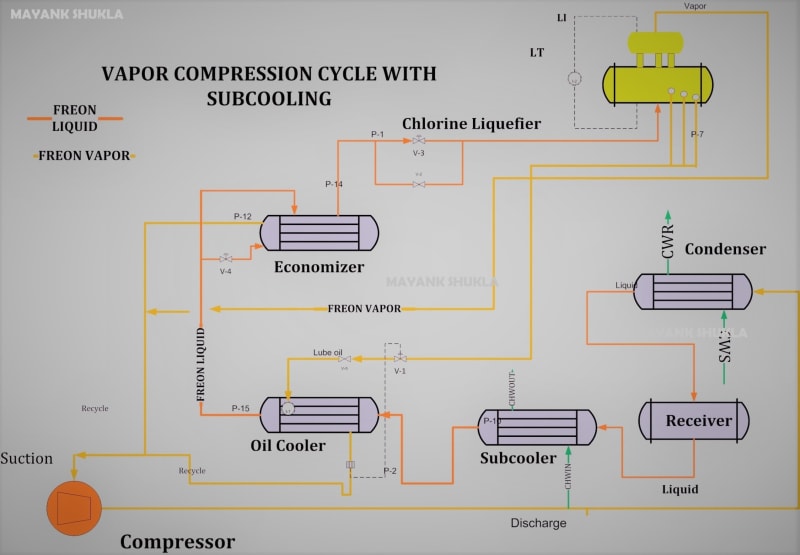
I have seen x2 refrigerants so far being used in Cl2 liquifier R22 & R-509. If the tubular exchanger has some leak ..say the tube side Cl2 oozes out what is the reaction with these refrigerants. I cannot find it.
Secondly...
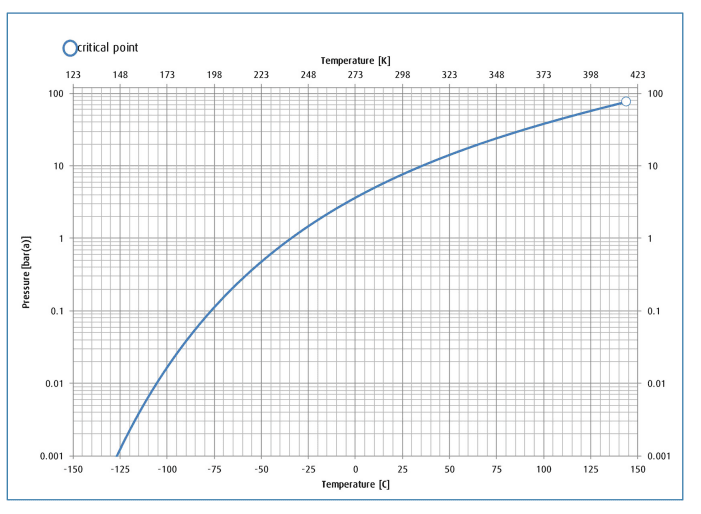
I know that Cl2 with humidity is enemy of carbon steel thats why we rigorously dry Cl2 (H2SO4 contact towers) before compression / liquefaction. Liquefiers tube / shell side is mostly made of of CS (or very rarely SS). These can be vertical (shell side Cl2 flows via gravity to storage, tube refrigerant) or inclined horizontal (tube side Cl2 flows via gravity to storage, shell refrigerant).
In a new company I have come across this design; tube side is Chilled water (closed circuit with glycol injection) & shell side Cl2. Cl2 pressure is to be higher than water to prevent water ingress into Cl2 (especially liquid Cl2). Do you think its safe or should they be using a refrigerant instead of water (the liquid Cl2 tank made of CS has 12ton cpacity)?
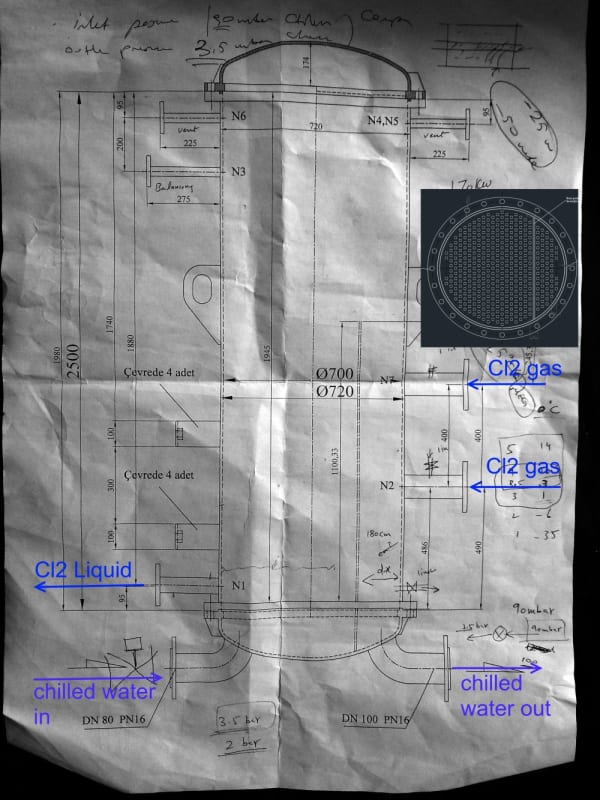
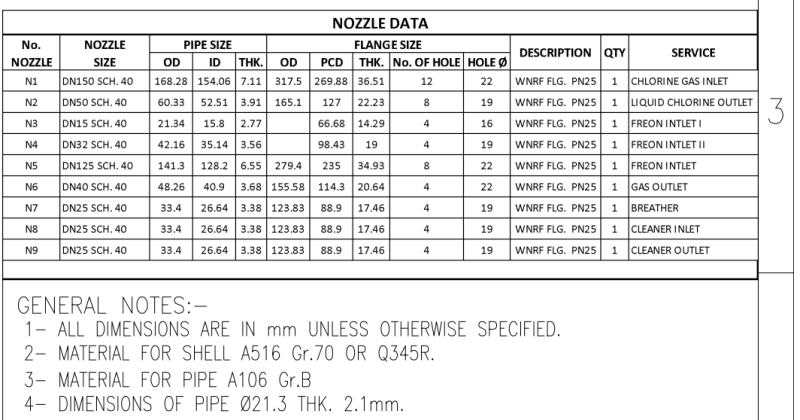
- Common inlet gas header with x2 inlet nozzles to exchanger
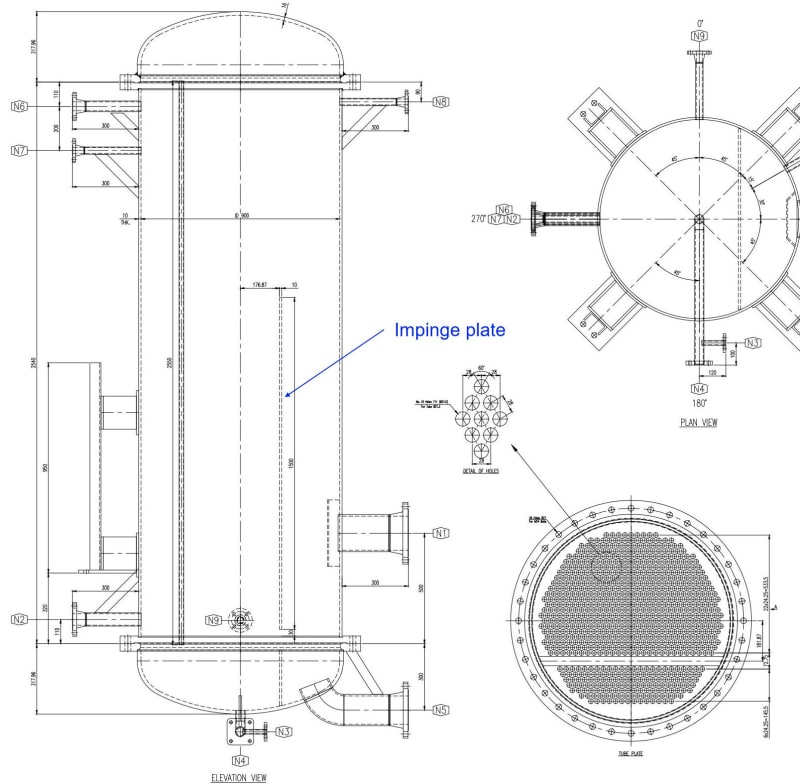
- x2 pass exchanger with 75% tubes for 1st pass & 25% tubes for 2nd pass (I dont know the reason)
- In the shell there is an impingement plate before feed Cl2 nozzles
https://www.linkedin.com/pulse/chlorine-liquefaction-performance-monitoring-benchmark-mayank-shukla/

I have seen x2 refrigerants so far being used in Cl2 liquifier R22 & R-509. If the tubular exchanger has some leak ..say the tube side Cl2 oozes out what is the reaction with these refrigerants. I cannot find it.
Secondly...
I know that Cl2 with humidity is enemy of carbon steel thats why we rigorously dry Cl2 (H2SO4 contact towers) before compression / liquefaction. Liquefiers tube / shell side is mostly made of of CS (or very rarely SS). These can be vertical (shell side Cl2 flows via gravity to storage, tube refrigerant) or inclined horizontal (tube side Cl2 flows via gravity to storage, shell refrigerant).
In a new company I have come across this design; tube side is Chilled water (closed circuit with glycol injection) & shell side Cl2. Cl2 pressure is to be higher than water to prevent water ingress into Cl2 (especially liquid Cl2). Do you think its safe or should they be using a refrigerant instead of water (the liquid Cl2 tank made of CS has 12ton cpacity)?

- Common inlet gas header with x2 inlet nozzles to exchanger
- x2 pass exchanger with 75% tubes for 1st pass & 25% tubes for 2nd pass (I dont know the reason)
- In the shell there is an impingement plate before feed Cl2 nozzles