Hello,
Let me establish foremost that I am extremely foreign to corrosion. I have a few questions concerning two methods of cathodic protection: sacrificial anodes and impressed current systems. Below is a summary of what I understand to be factual with respect to the process of corrosion and its prevention. Please let me know if any of the following is untrue, misleading, or a half-truth. I will also include explicit statements communicating my confusion on this topic. Thank you for your help!
My summary:
Every metal has an intrinsic electric potential associated with it. The electrode potential of a material is measured with respect to a standard hydrogen electrode defined at zero volts. Typically, a copper - copper sulfate reference is used for measurement of electrode potential due to its ease of production. For any given metal, the rate of cathodic reactions increase and the rate of anodic reactions decrease as the electrode potential of the metal becomes more negative. As electrode potential becomes more positive, the rate of cathodic reactions decrease and the rate of anodic reactions increase. Because anodic reactions include oxidation (rust), it is desired to keep the metal at a more negative electrode potential than its intrinsic range of potential. By supplying a DC electric current (via a rectifier) to the metal that is to be protected, the electrode potential of the metal is made more negative than its intrinsic range of potential, and thus corrosion of the structure is diminished.
My confusion concerning passive protection via a sacrificial anode:
Below is a picture of what I believe to be representative of passive protection of a structure buried in soil near a river. Again, please let me know if this is incorrect.
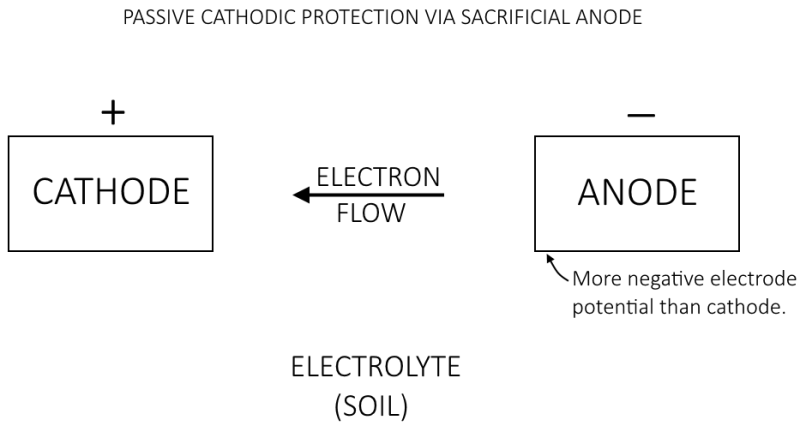
Why does the corrosion process prefer a metal with a more negative electrode potential?
My confusion concerning active protection via an impressed current system:
Below is a picture of what I believe to be representative of active protection of a structure buried in soil near a river.
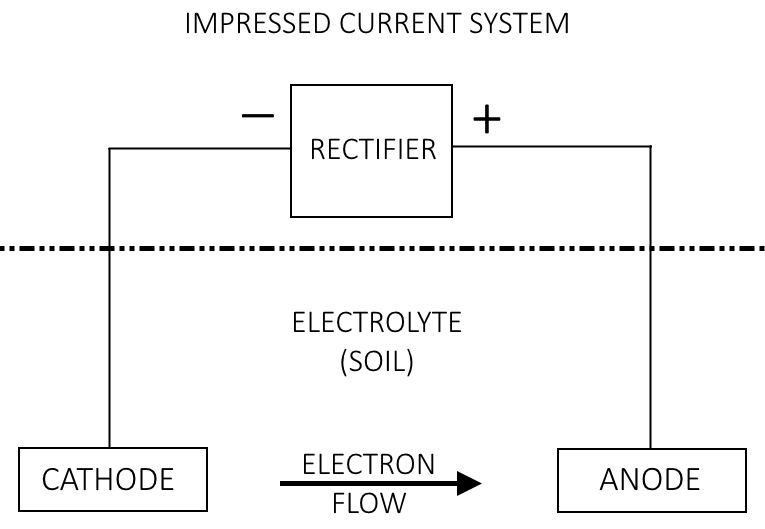
How does the cathode decrease in electrode potential when a current is applied via the rectifier? In contrast to the passive protection, electron flow is now away from the cathode. Is the difference between this method and the sacrificial anode method the fact that the source of electrons has changed? Would switching the polarities on the cathode and anode accelerate corrosion on the cathode instead of diminish it?
Let me establish foremost that I am extremely foreign to corrosion. I have a few questions concerning two methods of cathodic protection: sacrificial anodes and impressed current systems. Below is a summary of what I understand to be factual with respect to the process of corrosion and its prevention. Please let me know if any of the following is untrue, misleading, or a half-truth. I will also include explicit statements communicating my confusion on this topic. Thank you for your help!
My summary:
Every metal has an intrinsic electric potential associated with it. The electrode potential of a material is measured with respect to a standard hydrogen electrode defined at zero volts. Typically, a copper - copper sulfate reference is used for measurement of electrode potential due to its ease of production. For any given metal, the rate of cathodic reactions increase and the rate of anodic reactions decrease as the electrode potential of the metal becomes more negative. As electrode potential becomes more positive, the rate of cathodic reactions decrease and the rate of anodic reactions increase. Because anodic reactions include oxidation (rust), it is desired to keep the metal at a more negative electrode potential than its intrinsic range of potential. By supplying a DC electric current (via a rectifier) to the metal that is to be protected, the electrode potential of the metal is made more negative than its intrinsic range of potential, and thus corrosion of the structure is diminished.
My confusion concerning passive protection via a sacrificial anode:
Below is a picture of what I believe to be representative of passive protection of a structure buried in soil near a river. Again, please let me know if this is incorrect.

Why does the corrosion process prefer a metal with a more negative electrode potential?
My confusion concerning active protection via an impressed current system:
Below is a picture of what I believe to be representative of active protection of a structure buried in soil near a river.

How does the cathode decrease in electrode potential when a current is applied via the rectifier? In contrast to the passive protection, electron flow is now away from the cathode. Is the difference between this method and the sacrificial anode method the fact that the source of electrons has changed? Would switching the polarities on the cathode and anode accelerate corrosion on the cathode instead of diminish it?
