MortenA
Chemical
- Aug 20, 2001
- 2,998
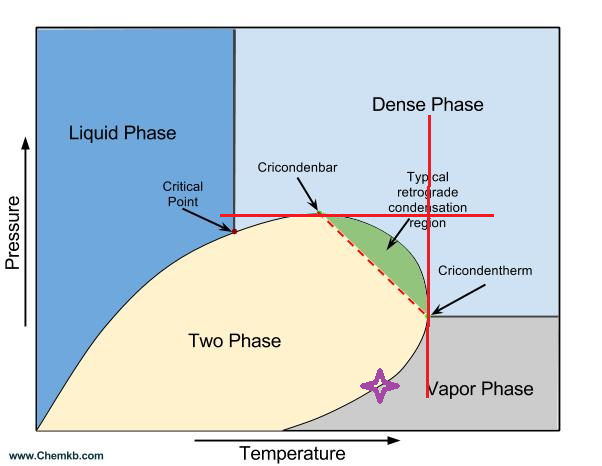
You get cricondenbar for pressure, cricondentherm for temperature, but what if its a PH diagram - then its a criconden???
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.