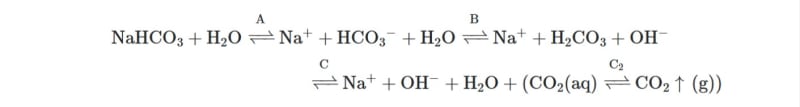I am wanting to confirm the impact on pH of sodium bisulphite being added to water. To do so, I need to calculate the number of H ions liberated when sodium bisulphite (NaHSO3) is added to water. For example, I know for ferric chloride the reaction is
FeCl3+3H20 ⇌ Fe(OH)3+ 3H+ + 3Cl-
i.e. 3 Hs and acidity is released. What is the equivalent reaction for NaHSO3 and H20? Thanks
FeCl3+3H20 ⇌ Fe(OH)3+ 3H+ + 3Cl-
i.e. 3 Hs and acidity is released. What is the equivalent reaction for NaHSO3 and H20? Thanks