Hi there,
I've been reading a bit in the forum and internet about the galvanic corrosion between aluminium.
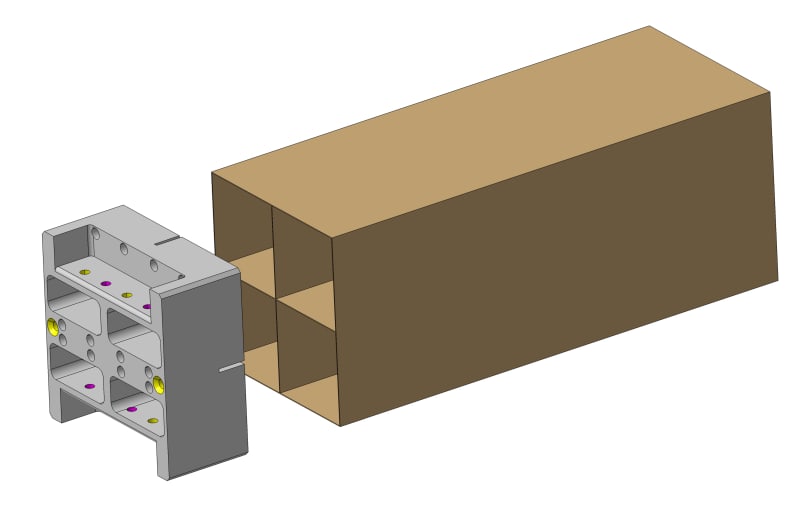
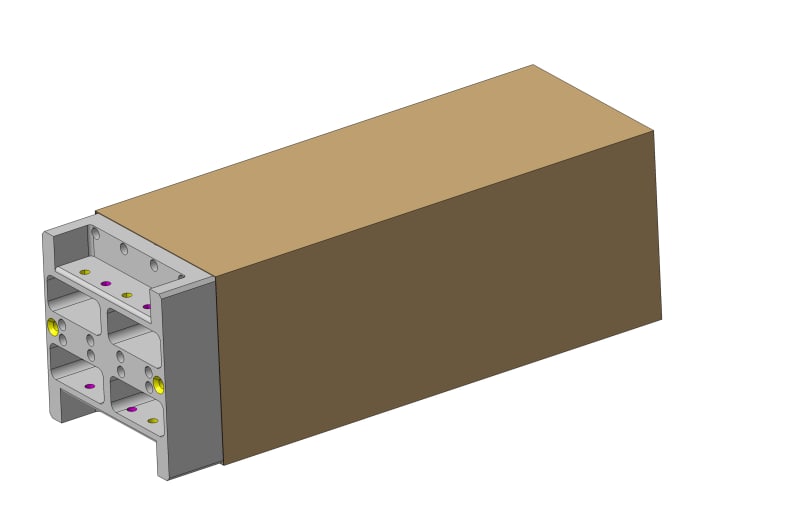
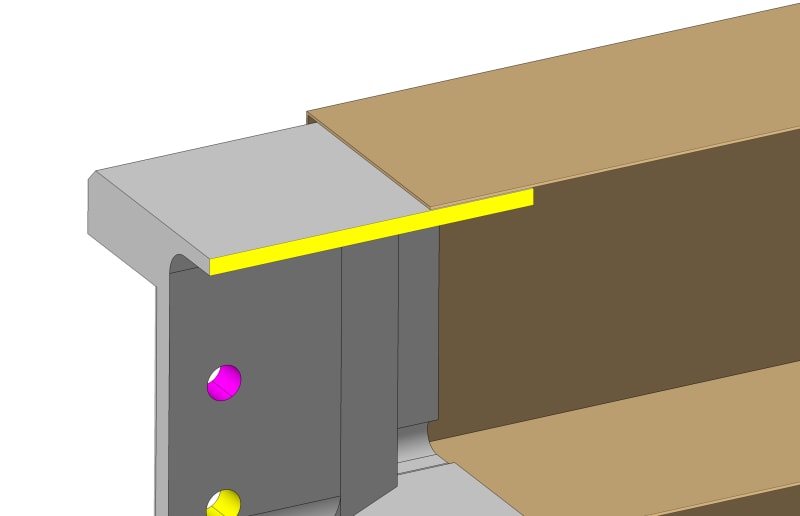
We have a carbon fiber reinforced epoxy boxes with a load and they are hung in one end. We glue an aluminium insert. The gap is 150 microns around and the glue 3M DP 410.
In theory, there is no direct contact between the carbon fiber and the aluminium, since we have the glue everywhere around the aluminium.
We can't use glass fiber in between.
I am not familiarized with the treatments but there is a company here close by which can do a chromate (Alodine) the aluminium parts.
Do you think this is safe enough to avoid the galvanic corrosion?
thanks
regards
I've been reading a bit in the forum and internet about the galvanic corrosion between aluminium.
We have a carbon fiber reinforced epoxy boxes with a load and they are hung in one end. We glue an aluminium insert. The gap is 150 microns around and the glue 3M DP 410.
In theory, there is no direct contact between the carbon fiber and the aluminium, since we have the glue everywhere around the aluminium.
We can't use glass fiber in between.
I am not familiarized with the treatments but there is a company here close by which can do a chromate (Alodine) the aluminium parts.
Do you think this is safe enough to avoid the galvanic corrosion?
thanks
regards