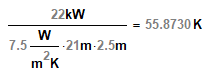Hi all,
I am more a pressure vessel engineer, but I am trying to do a pre-design check on the heating taking place in a compressor room. I am trying to figure out if cooling of the room is needed as the intake air temperature should not exceed 46deg C.
My Heat Transfer/Thermodynamics is very rusty, but I tried anyway and now my answer seems way too low so I expect I made a mistake somewhere.
Details are:
2 air compressors each 22kW
Outside Air temp 32deg C
Altitude above sealevel 1500m
Room size 6m x 4.5m by 2.5m high
Assuming almost all energy is converted to heat, I used Q=cmΔT to calculate delta T (the expected temperature change) but I got an answer of 0.93deg C which seems too low...
Can someone please assist?
I am more a pressure vessel engineer, but I am trying to do a pre-design check on the heating taking place in a compressor room. I am trying to figure out if cooling of the room is needed as the intake air temperature should not exceed 46deg C.
My Heat Transfer/Thermodynamics is very rusty, but I tried anyway and now my answer seems way too low so I expect I made a mistake somewhere.
Details are:
2 air compressors each 22kW
Outside Air temp 32deg C
Altitude above sealevel 1500m
Room size 6m x 4.5m by 2.5m high
Assuming almost all energy is converted to heat, I used Q=cmΔT to calculate delta T (the expected temperature change) but I got an answer of 0.93deg C which seems too low...
Can someone please assist?