Ander Azpi
Student
- Mar 3, 2021
- 7
Hello,
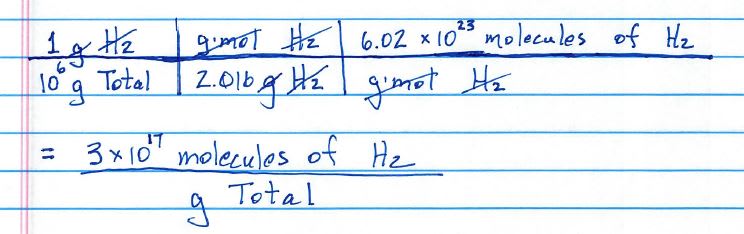
I am struggling when trying to convert 1wppm of H2 into another unit (that is the concentration of hydrogen I receive).
I've found some information about ppm, but I'm not sure how to do the wppm (weight parts per million I assume) conversion.
Thanks in advance
I am struggling when trying to convert 1wppm of H2 into another unit (that is the concentration of hydrogen I receive).
I've found some information about ppm, but I'm not sure how to do the wppm (weight parts per million I assume) conversion.
Thanks in advance