ReuvenD10
Mechanical
- Oct 15, 2020
- 6
hello everybody,
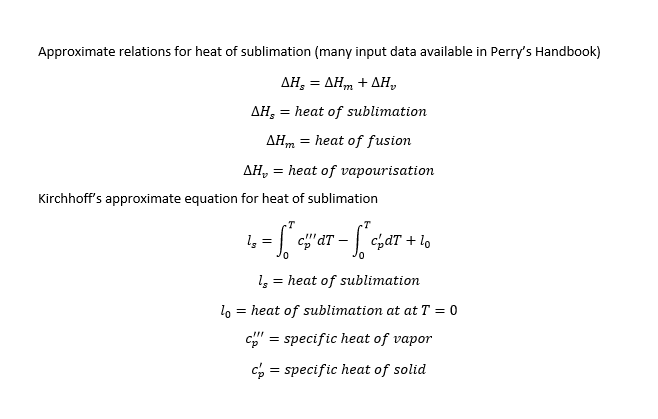
I try to calculate the latent heat of the sublimation process. I google for formula or table/graph and I didn't find.
somebody can help with this?
( I need the latent heat to determine the sublimation time in 30,000 feet altitude, aircraft field )
Thanks.
I try to calculate the latent heat of the sublimation process. I google for formula or table/graph and I didn't find.
somebody can help with this?
( I need the latent heat to determine the sublimation time in 30,000 feet altitude, aircraft field )
Thanks.