Hi,
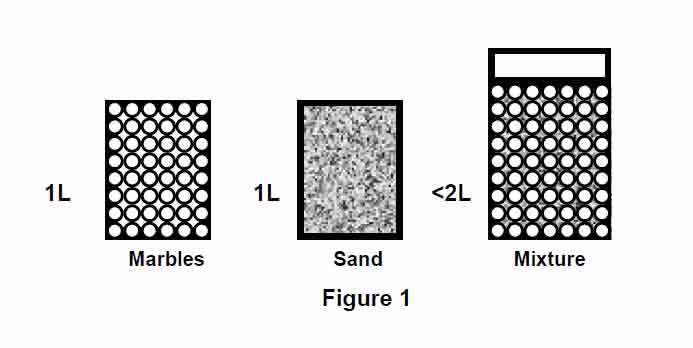
I am doing a pressure drop calculation for my project where there is a mixing of two fluids having densities of 980 Kg/m3 & 850 Kg/m3. Flow rates are 400 m3/hr & 120 m3/hr respectively. I am unsure of what the outlet flow rate will be. If we consider the volumetric flow rate, the outlet should be 520 m3/hr, but if we convert these into mass flow, then the flow rate will be 392000 + 102000 = 494000 Kg/hr which comes to 504 m3/hr. So how should I calculate the outlet flow rate & what will be the density of the mixed fluid that I should consider as this will also impact the pressure drop.
I am doing a pressure drop calculation for my project where there is a mixing of two fluids having densities of 980 Kg/m3 & 850 Kg/m3. Flow rates are 400 m3/hr & 120 m3/hr respectively. I am unsure of what the outlet flow rate will be. If we consider the volumetric flow rate, the outlet should be 520 m3/hr, but if we convert these into mass flow, then the flow rate will be 392000 + 102000 = 494000 Kg/hr which comes to 504 m3/hr. So how should I calculate the outlet flow rate & what will be the density of the mixed fluid that I should consider as this will also impact the pressure drop.