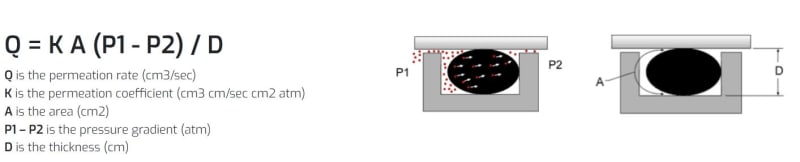
I'm looking at the figures in The greater the thickness in the permeation direction the smaller the permeation rate, right?
I think the D dimension should be at right angles (horizontal) to what is shown.
----------------------------------------
The Help for this program was created in Windows Help format, which depends on a feature that isn't included in this version of Windows.
I think the D dimension should be at right angles (horizontal) to what is shown.
----------------------------------------
The Help for this program was created in Windows Help format, which depends on a feature that isn't included in this version of Windows.