Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ph drop after reverse osmosis
- Thread starter me2016
- Start date
- Status
- Not open for further replies.
It will depend on the chemistry of the water that you are treating and you have not given much information.
But typically its caused by the carbon dioxide in solution. Two things happen to cause the pH drop.
The CO2 already in solution in the feed water will pass straight through the membrane into the permeate or product water with very little going out with the concentrate. Secondly the RO process removes most of the alkalinity which buffers the acidic effect of the CO2 in solution which is in the form of carbonic acid. With less alkalinity to buffer the carbonic acid the pH drops.
This typically happens where the feed water is less than about 8.0 ph. Plants that have feedwater pHs above 8 have little or no reduction in pH because no CO2 remains in solution at that pH.
Regards
Ashtree
"Any water can be made potable if you filter it through enough money"
But typically its caused by the carbon dioxide in solution. Two things happen to cause the pH drop.
The CO2 already in solution in the feed water will pass straight through the membrane into the permeate or product water with very little going out with the concentrate. Secondly the RO process removes most of the alkalinity which buffers the acidic effect of the CO2 in solution which is in the form of carbonic acid. With less alkalinity to buffer the carbonic acid the pH drops.
This typically happens where the feed water is less than about 8.0 ph. Plants that have feedwater pHs above 8 have little or no reduction in pH because no CO2 remains in solution at that pH.
Regards
Ashtree
"Any water can be made potable if you filter it through enough money"
?? pH is the negative of the logarithm of the hydrogen activity
Don't know about alkaline water, but DI water with 18-megohm resistivity will dissolve CO2 from the air and eventually become sufficient acidic to etch susceptible materials, like glass
TTFN (ta ta for now)
I can do absolutely anything. I'm an expert! faq731-376 forum1529
Don't know about alkaline water, but DI water with 18-megohm resistivity will dissolve CO2 from the air and eventually become sufficient acidic to etch susceptible materials, like glass
TTFN (ta ta for now)
I can do absolutely anything. I'm an expert! faq731-376 forum1529
When CO2 is mixed into water, the CO2 ionizes to form carbonic acid:
H2O + CO2 ----> H2CO3
H2CO3 ----> H+ + HCO3 -
You are correct that the H+ is the measure of acidity. However, notice also that alkalinity (HCO3) may now be present.

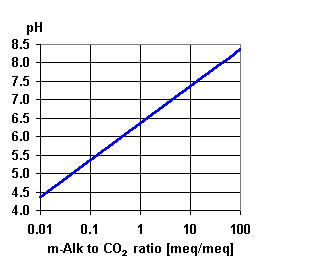
People that work in the water business are quite familiar with the ratio of alkalinity to carbon dioxide. Further information from Nalco, a water treatment company.
Carbon dioxide is what is known as a weak acid which means that the pH of a carbon dioxide saturated solution will never drop below a pH of approximately 4.3 and therefore will never be capable of etching glass. Strong acids such as hydrogen fluoride are used to etch glass.
You are getting close to being a AGW proponent with your comment that carbon dioxide is being absorbed from the atmosphere into water (the ocean).
Every atmospheric gas is in equilibrium with that gas dissolved in ocean water. The oceans are a major sink for atmospheric carbon dioxide. However, the oceans are no longer able to absorb the increased concentration of CO2 in the atmosphere without changes to the acidity levels.
H2O + CO2 ----> H2CO3
H2CO3 ----> H+ + HCO3 -
You are correct that the H+ is the measure of acidity. However, notice also that alkalinity (HCO3) may now be present.

People that work in the water business are quite familiar with the ratio of alkalinity to carbon dioxide. Further information from Nalco, a water treatment company.
Carbon dioxide is what is known as a weak acid which means that the pH of a carbon dioxide saturated solution will never drop below a pH of approximately 4.3 and therefore will never be capable of etching glass. Strong acids such as hydrogen fluoride are used to etch glass.
You are getting close to being a AGW proponent with your comment that carbon dioxide is being absorbed from the atmosphere into water (the ocean).
Every atmospheric gas is in equilibrium with that gas dissolved in ocean water. The oceans are a major sink for atmospheric carbon dioxide. However, the oceans are no longer able to absorb the increased concentration of CO2 in the atmosphere without changes to the acidity levels.
- Status
- Not open for further replies.
Similar threads
- Replies
- 3
- Views
- 14
- Locked
- Question
- Replies
- 4
- Views
- 11
- Replies
- 0
- Views
- 3
- Replies
- 9
- Views
- 14
- Replies
- 8
- Views
- 10