Hi community!
sorry, this may be a stupid question and not sure where to post it..
and not sure where to post it..
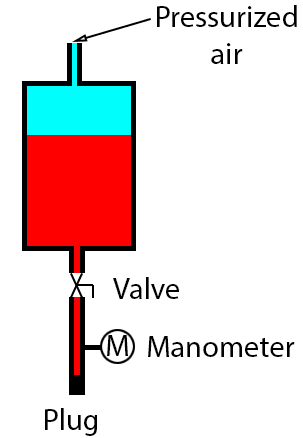
I have the following case: a tank with a pipe connected to its bottom, closed with a plug is partially filled with a liquid (red); the tank is pressurized with compressed air (light blue) from its top, let's say at 8 bar.
Valve at the bottom is opened, so the pipe is filled with liquid.
In this situation the manometer will read 8 bar.
Now, I'm closing the valve and I'm depressurizing the tank from the top: I have atmospheric pressure in the tank now.
What value the manometer will read? I think still 8 bar?
sorry, this may be a stupid question
I have the following case: a tank with a pipe connected to its bottom, closed with a plug is partially filled with a liquid (red); the tank is pressurized with compressed air (light blue) from its top, let's say at 8 bar.
Valve at the bottom is opened, so the pipe is filled with liquid.
In this situation the manometer will read 8 bar.
Now, I'm closing the valve and I'm depressurizing the tank from the top: I have atmospheric pressure in the tank now.
What value the manometer will read? I think still 8 bar?

