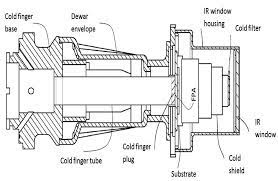
I'm in a design concept stage at the moment. I have a Stirling Cooler which will be running at -120c. The head of the cooler will be enclosed with a box. At this temperature carbon dioxide and water will instantly freeze on the surface and form a large spherical ice ball. I'm looking at ways to prevent this ice build up.
At the moment my ideas are as follows.
[ul][li]Seal it from the otherside using o-rings and sealant tape, them pump the inside of the chamber with nitrogen or some other dry gas.[/li]
[li]Seal it from the outside and create a vacuum.[/li]
[li]Fill any cavities with a physical foam (Although at such low temperatures the foam may break down).[/li][/ul]
I did look at some omniphobic coating, but I doubt they would be robust enough to handle repelling moisture at such low levels. Can does anyone have any other ideas?
Thanks
At the moment my ideas are as follows.
[ul][li]Seal it from the otherside using o-rings and sealant tape, them pump the inside of the chamber with nitrogen or some other dry gas.[/li]
[li]Seal it from the outside and create a vacuum.[/li]
[li]Fill any cavities with a physical foam (Although at such low temperatures the foam may break down).[/li][/ul]
I did look at some omniphobic coating, but I doubt they would be robust enough to handle repelling moisture at such low levels. Can does anyone have any other ideas?
Thanks