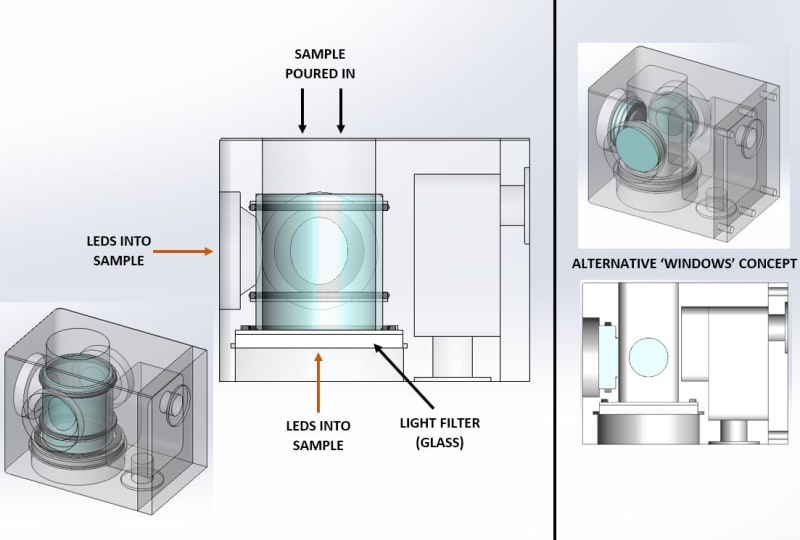
I'm designing a system where a sea water sample must have its temperature controlled between near its freezing point (-2°C) and +30°C to simulate sea temperatures. To control it, the sample will be in contact with a block, which will be controlled by an external circulating water bath. The image should be useful.
I’m new to the world of temperature control and heat exchange, so please correct me here.
MATERIAL SELECTION:
- I believe thermal conductivity to be the most influential factor, but have read up on heat capacity and a combination of the two – thermal resistivity. Are these other two worth considering here?
- Sea water, therefore, corrosive environment
- SELECTION: It seems aluminiums and coppers are typical used in water cooling system, but the sea water element leads me to the route of bronze, copper-nickel and titanium (based on wiki page, which I now can’t locate). Any experience of material selection?
DESIGN:
- I understand that it would be ideal to have as much of the sample in contact with the heat exchange block, but I must stick with a system using a glass tube as indicated.
- Air-cooled heat sinks have fins – do I want to go down a similar route of maximising surface area of block with water bath flow? I could add a number of drilled holes in that section.
- Should the volume of the water bath chamber be maximised?
- The block itself may be both smaller and larger in terms of volume of material. What pros/cons do they have? A large block would take longer to change temperature surely, meaning I want the block to be as small in volume.
- Worth going down the route of simulations?
Thank you in advance!
I’m new to the world of temperature control and heat exchange, so please correct me here.
MATERIAL SELECTION:
- I believe thermal conductivity to be the most influential factor, but have read up on heat capacity and a combination of the two – thermal resistivity. Are these other two worth considering here?
- Sea water, therefore, corrosive environment
- SELECTION: It seems aluminiums and coppers are typical used in water cooling system, but the sea water element leads me to the route of bronze, copper-nickel and titanium (based on wiki page, which I now can’t locate). Any experience of material selection?
DESIGN:
- I understand that it would be ideal to have as much of the sample in contact with the heat exchange block, but I must stick with a system using a glass tube as indicated.
- Air-cooled heat sinks have fins – do I want to go down a similar route of maximising surface area of block with water bath flow? I could add a number of drilled holes in that section.
- Should the volume of the water bath chamber be maximised?
- The block itself may be both smaller and larger in terms of volume of material. What pros/cons do they have? A large block would take longer to change temperature surely, meaning I want the block to be as small in volume.
- Worth going down the route of simulations?
Thank you in advance!