Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
The thermal heat capacity for flue gases around 78 kj/kg °cAt a simple level it's just a massive equation once you know thermal heat capacity of your flue gas.
But 150C looks very ambitious.
You then need to know start temp of your boiler feed water and calculate steam production. Then allow something for losses.
This is a very simplistic approach, but then you've not given any details.
It might be a lot less steam than you think....
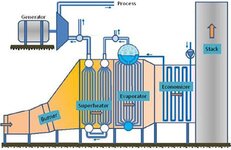
It can be saturated steam i just need the process to know the quantity of heat can be used from flue gases at temp 900 °c and quantity of steam can be generatedGenerate steam at what pressure? Does steam product have to superheated - to what temp ?
When i calculated it this way the results were far too much to be possibleThe heat available from the flue gas is
Q = m Cp dT
I don't calculate in SI units but in consistent units I believe this would be
Q = KJ/hr total heat available in flue gas
m = kg/hr mass flowrate of flue gas
Cp = KJ/(kg C) specific heat of flue gas
dT = change in temperature of flue gas in deg. C
If your steam has an enthalpy of vaporization of 2260 kJ/kg as you indicated above then total steam mass vaporized is:
m = Q/2260
where mass is in kg/hr of steam, Q is the heat available calculated above and 2260 is the latent heat of vaporization of the steam.
The key is your dT which at 900 to 150 looks unfeasible without having a HUGE exchanger. Plus losses in terms of heat etc.The heat available from the flue gas is
Q = m Cp dT
I don't calculate in SI units but in consistent units I believe this would be
Q = KJ/hr total heat available in flue gas
m = kg/hr mass flowrate of flue gas
Cp = KJ/(kg C) specific heat of flue gas
dT = change in temperature of flue gas in deg. C
If your steam has an enthalpy of vaporization of 2260 kJ/kg as you indicated above then total steam mass vaporized is:
m = Q/2260
where mass is in kg/hr of steam, Q is the heat available calculated above and 2260 is the latent heat of vaporization of the steam.