-
1
- #1
Martinos
Mechanical
- Nov 12, 2014
- 52
Hi dudes.
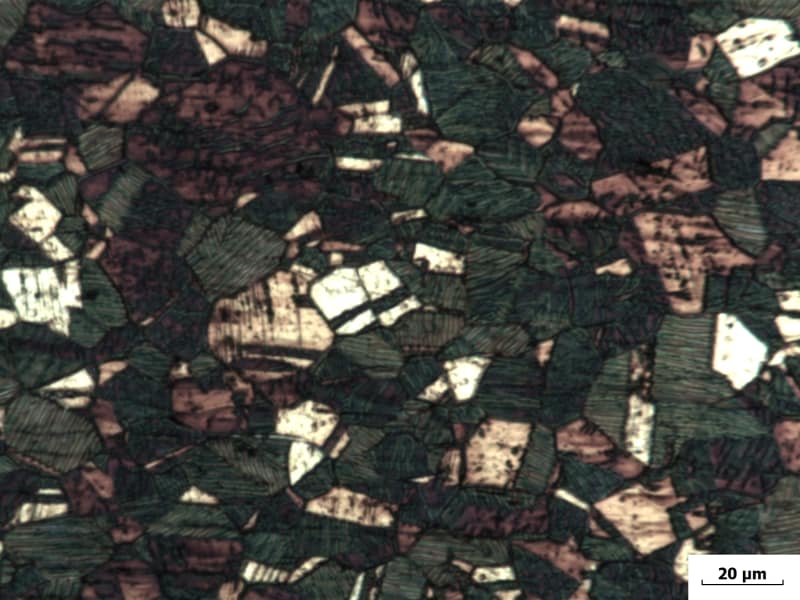
I have here drawn bar made of 304Cu (1.4567) steel in solution annealed condition. Chemical composition C 0.011 Si 0.40 Mn 0.75 S 0.02 Cr 17.1 Mo 0.32 Ni 8.6 Cu 3.1%.
Please take a look at a photomicrographs linked below (1x transverse cut, 2x longitudinal cut). I have a dispute regarding prolonged stringers. May it be deformation induced martensite? My first conclusion was delta ferrite but now I am not sure about it.
Thank you!
Link
Link
Link
I have here drawn bar made of 304Cu (1.4567) steel in solution annealed condition. Chemical composition C 0.011 Si 0.40 Mn 0.75 S 0.02 Cr 17.1 Mo 0.32 Ni 8.6 Cu 3.1%.
Please take a look at a photomicrographs linked below (1x transverse cut, 2x longitudinal cut). I have a dispute regarding prolonged stringers. May it be deformation induced martensite? My first conclusion was delta ferrite but now I am not sure about it.
Thank you!
Link
Link
Link