Mechanical_weld
Mechanical
- May 25, 2020
- 5
Has anyone met?
Is it possible use 316L steel for 5%HCl; Temp:ambient;~20C
Will it be resistant?
Is it possible use 316L steel for 5%HCl; Temp:ambient;~20C
Will it be resistant?
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
All kinds and varieties of stainless steels become active and are attacked by hydrochloric acid. In very low concentrations, the acid can cause pitting, crevice corrosion or CSCC. With duplex grades, the tendency is to attack the ferrite phase preferentially. This phenomenon is also observed in weldments in some cases.
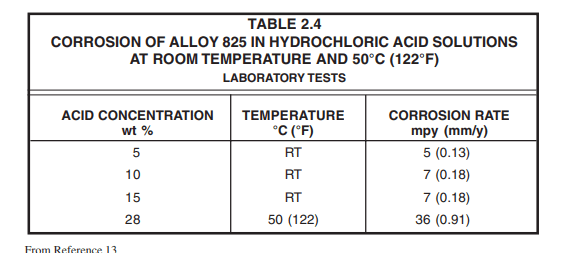
Alloy 825 and Alloy 20 resist corrosion at all concentrations at temperatures <40 °C (100 °F).
The 6% Mo superaustenitic stainless steels, such as UNS S31254, UNS N08367, and UNS N08926, can be used in some applications in hydrochloric acid concentrations < 3 wt%. The 7% Mo superaustenitic stainless steel UNS S32654 with nominal 7.3% Mo can be used up to about 8% acid at room temperature.