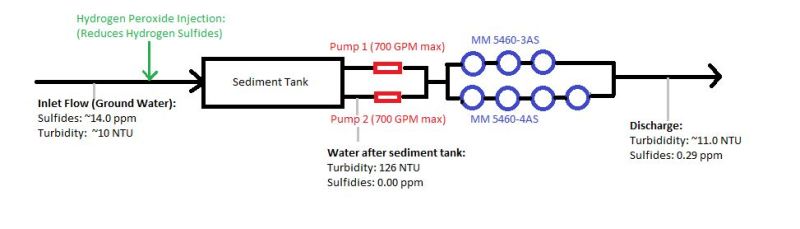
I apologize if this has been discussed already. I was sifting through the posts for a while, and I unable to find anything similar to my problem.
We have two sediment tanks both have large volumes (30,000 gal together), Depending on the in fluent flow (which varies), the residence time in the tanks can be anywhere around 50-80 minutes.
The average concentration of the sulfides coming in is 15-20 ppm. The inflow ranges from 500-600 gal/min on an average day. We use a total sulfides kit using a dropper method, to eyeball the approx concentration.
After the filters we use a Colorimeter (la Motte), which is able to detect sulfides at low levels (<1.5 ppm). I also use this to test the effluent coming from the sediment tanks (which is usually 0.00 ppm). Every once in a while our peroxide pump will get air locked overnight (maybe once every two months or so), so the hydrogen sulfide isn't reduced over night and enter the filters, not sure if this has anything to do with it.
I've attached a picture of our process/setup.
A little more input on the filters: They are multi media filters with anthracite, fine and course garnet. My predecessor was not operating these filters correctly. Inside the second sediment tank, we have two float switches that were connected to two different pumps. Normally only one pump would turn and if the influent flow rose to higher levels, the 2nd pump would kick on. The problem with this is how the backwash works with these filters. There is a restrictor valve on the filters. You would adjust the valve to the maximum flow before seeing media loss in the backwash effluent. So if my predecessor calibrated the restrictor valve based on the flow of one pump turning on (700 GPM), then if two pumps turned on (1400 GPM) chances are we be losing media (the backwash flow would double). Or vise-versa, if he had calibrated the valve to two pumps turning on (which I believe to be the case), then every time the backwash sequence started with only one pump (which was usually the case, maybe 90% of the time) then the backwash would only be half of what it should be (meaning it wasn't cleaning much at all). So this went on for several months I'd imagine before I joined. Sure enough we had a pressure differential problem. The filters were getting massively clogged (massive flow loss). To remedy this I was able to get both pumps on one float switch (one constant flowrate to adjust the restrictor valve), gave each media pod a chlorine shock, and increased the backwash to the recommended amount. I have ran these filters like this for several weeks now, and the sulfides "reappearing" problem is still occurring.
Thanks for the reply, If you need more information just let me know. Any help/direction you can give would be very much appreciated.