Hello,
I am working on a drinking water treatment project design which takes place in an African country.
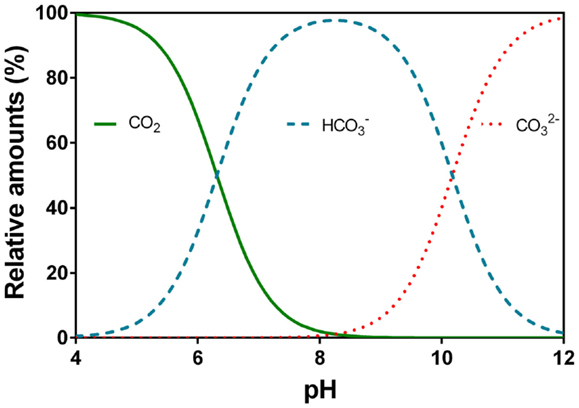
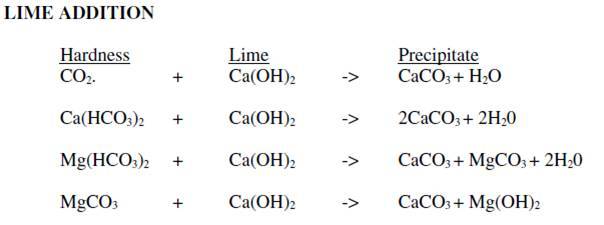
The chosen surface water (and only available) is soft and very low in mineral content (very low alkalinity). Alkalinity is not enough for the coagulant additiv to be effective (generaly Aluminum sulfate). By working with the "Hallopeau & Dubin carbonic graph", I realize adding soda ash (Na2CO3) or lime would not solve the problem as we quickly arrive on the vertical part of the curves where pH rises but no more the alkalinity.
If I do the calculation properly (using the graph), adding soda ash or lime would allow me to use effectivly 25 mg/l aluminum sulfate. All additional aluminum sulfate would not have alkalinity to consume. I know by experience that in rainy season, I could need to use until 80 to 100 mg/l aluminum sulfate to reach an effective coagulation, so 25 mg/l is not enough.
All the ferric coagulants consume also alkalinity so changing coagulant additiv to a ferric coagulant does not seem to me a good solution.
Another alternative is to add carbonic gaz CO2 jointly with lime or soda ash, but this requires:
- supply of carbonic gaz; in deep african part, probably difficult;
- refregirated storage of carbonic gaz; in an environment where there is often power outage, risky;
- highly qualified operators which lack in that area;
Therefore it seems to me unappropriate to implement such process considering the above difficulties and risks.
Finally, I read it was also possible to mineralize water through slow carbonate dissolution using marine limestone but it seems this process is adapted to small treatment plants (500 m3/d maximum) whereas the expected treatment plant capacity in this project is 11 000 m3/d.
I did not find an appropriate solution to solve the very low alkalinity problem of this water. I would really appreciate if someone could help me on this !
Here below are some caracteristics of the water measured end of february which corresponds to the end of the dry season:
- Water temperature: 25°C
- pH: 6
- Complete alcalimetric title (CAT): 0.2 °F and Calcium title (TCa): 0.4°F
- Turbidity: 20 NTU
- Dissolved suspended solids: 16 mg/l
- Color: 115 (Pt)
- Permanganate oxidability: 8.6 mg/l
- Total iron: 0.85 mg/l
Thank you very much in advance for your help !
Arnaud
I am working on a drinking water treatment project design which takes place in an African country.
The chosen surface water (and only available) is soft and very low in mineral content (very low alkalinity). Alkalinity is not enough for the coagulant additiv to be effective (generaly Aluminum sulfate). By working with the "Hallopeau & Dubin carbonic graph", I realize adding soda ash (Na2CO3) or lime would not solve the problem as we quickly arrive on the vertical part of the curves where pH rises but no more the alkalinity.
If I do the calculation properly (using the graph), adding soda ash or lime would allow me to use effectivly 25 mg/l aluminum sulfate. All additional aluminum sulfate would not have alkalinity to consume. I know by experience that in rainy season, I could need to use until 80 to 100 mg/l aluminum sulfate to reach an effective coagulation, so 25 mg/l is not enough.
All the ferric coagulants consume also alkalinity so changing coagulant additiv to a ferric coagulant does not seem to me a good solution.
Another alternative is to add carbonic gaz CO2 jointly with lime or soda ash, but this requires:
- supply of carbonic gaz; in deep african part, probably difficult;
- refregirated storage of carbonic gaz; in an environment where there is often power outage, risky;
- highly qualified operators which lack in that area;
Therefore it seems to me unappropriate to implement such process considering the above difficulties and risks.
Finally, I read it was also possible to mineralize water through slow carbonate dissolution using marine limestone but it seems this process is adapted to small treatment plants (500 m3/d maximum) whereas the expected treatment plant capacity in this project is 11 000 m3/d.
I did not find an appropriate solution to solve the very low alkalinity problem of this water. I would really appreciate if someone could help me on this !
Here below are some caracteristics of the water measured end of february which corresponds to the end of the dry season:
- Water temperature: 25°C
- pH: 6
- Complete alcalimetric title (CAT): 0.2 °F and Calcium title (TCa): 0.4°F
- Turbidity: 20 NTU
- Dissolved suspended solids: 16 mg/l
- Color: 115 (Pt)
- Permanganate oxidability: 8.6 mg/l
- Total iron: 0.85 mg/l
Thank you very much in advance for your help !
Arnaud