Sirius P.Eng.
Chemical
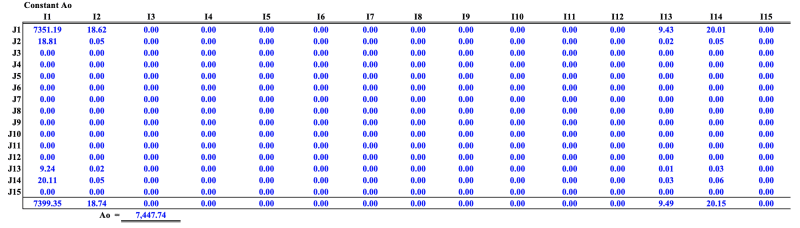
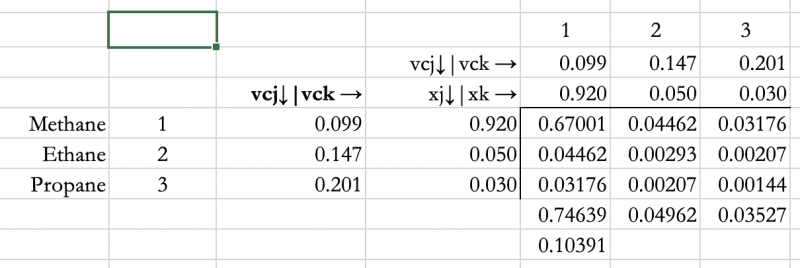
I'm currently applying the Lee-Kesler EoS to calculate enthalpy departure but I have run into problems applying the mixing rules mostly because different approaches are used in literature.
All the other formulas (Zci, vci and w and Pc) are straightforward except the critical molar volume (Vc) and critical temperature (Tc).
I'm having challenges simplifying this formula for a single has natural gas composition. How do I simplify the equations for a natural gas stream with no liquid phase. I will be glad if anyone with experience with applying the Lee-Kesler EoS mixing rules can shed some light on this.
I have attached screenshots of the mixing rules from theAll the other formulas are straightforward.
All the other formulas (Zci, vci and w and Pc) are straightforward except the critical molar volume (Vc) and critical temperature (Tc).
I'm having challenges simplifying this formula for a single has natural gas composition. How do I simplify the equations for a natural gas stream with no liquid phase. I will be glad if anyone with experience with applying the Lee-Kesler EoS mixing rules can shed some light on this.
I have attached screenshots of the mixing rules from theAll the other formulas are straightforward.