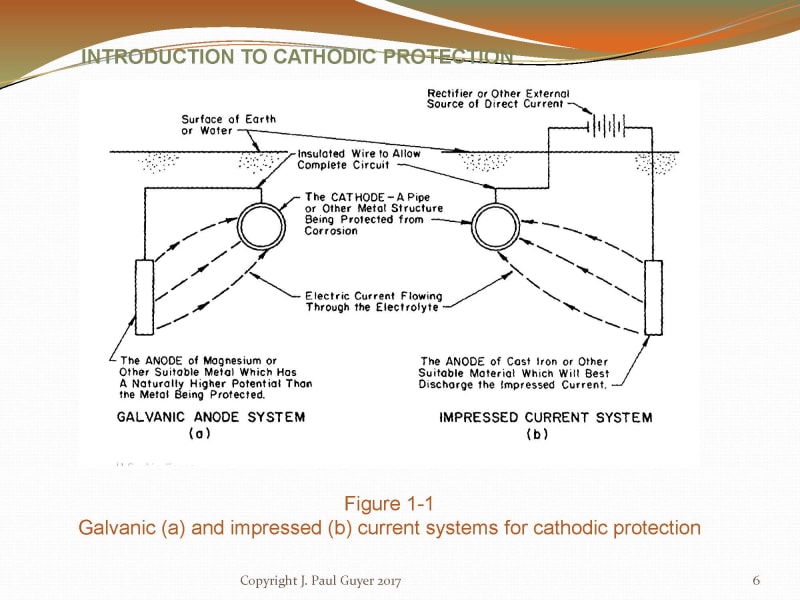
My question if for galvanic systems only (not immersed).
Pardon my ignorance, but in the first image, the theory dictates an insulated wire back from the sacrificial anode to the tank, while in the second image, the anodes seems directly welded to the tank, visibly by a conducting metal, and finally in the bottom image, clearly an impressed system, we see the wiring.
What is the correct method? Am I missing anything?
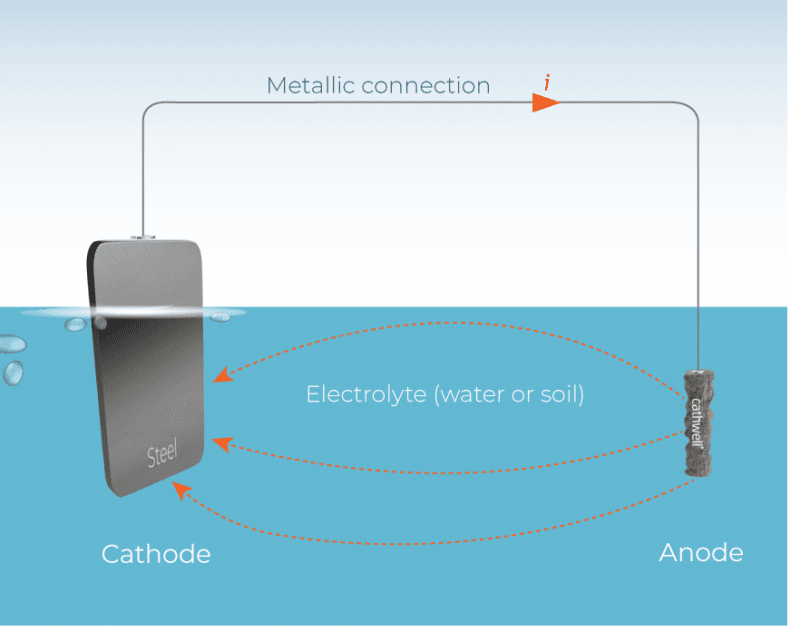

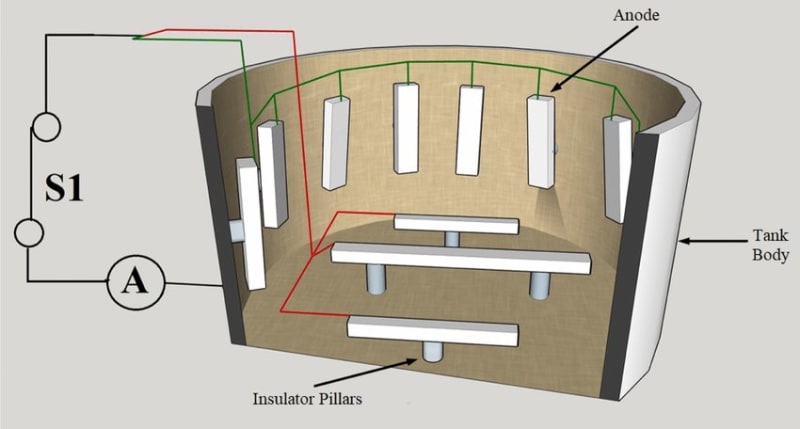
Pardon my ignorance, but in the first image, the theory dictates an insulated wire back from the sacrificial anode to the tank, while in the second image, the anodes seems directly welded to the tank, visibly by a conducting metal, and finally in the bottom image, clearly an impressed system, we see the wiring.
What is the correct method? Am I missing anything?