LSUengr2013
Civil/Environmental
- Jul 20, 2020
- 13
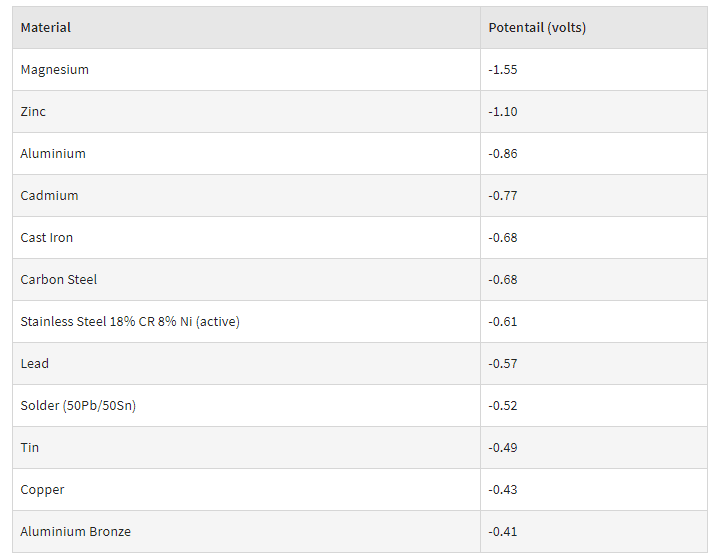
I am having to install an aluminum beam between two steel beams and I have read some conflicting information regarding galvanic corrosion.
Tried to upload some images but doesn't seem to be working. I was proposing to use a galvanized double clip angle to bolt the aluminum beam into the existing steel ones. This was based on the Aluminum Steel Guide 2005 Section 6.7.
"Where aluminum is in contact with or fastened to the materials specified in Sections 6.7.1 through 6.7.3, direct contact between the aluminum and the other material shall be prevented as specified in those sections or by placing a compatible, nonporous isolator between the aluminum and the other material.
6.7.1 Steel
Steel surfaces to be placed in contact with uncoated aluminum shall be painted with a coating suitable for the service. Where very corrosive conditions are expected, additional protection can be obtained by applying a sealant that excludes moisture from the joint during service. Aluminized, hot-dip galvanized or electro-galvanized steel in contact with aluminum need not be painted. Stainless steel (300 series) in contact with aluminum need not be painted except in high chloride environments"
But the more I have dug into the subject it doesn't seem like just having the steel elements galvanized is enough precaution. This will be an indoor application, but large garage doors open it up to quite high levels of humidity at times.
I've also found the suggestion of painting any dissimilar contact surface with one coat of zinc chromate primer and then one heavy coat of aluminum pigmented asphalt paint.
If anyone has any insight they could share to the best path forward the better. Thanks in advace.
Tried to upload some images but doesn't seem to be working. I was proposing to use a galvanized double clip angle to bolt the aluminum beam into the existing steel ones. This was based on the Aluminum Steel Guide 2005 Section 6.7.
"Where aluminum is in contact with or fastened to the materials specified in Sections 6.7.1 through 6.7.3, direct contact between the aluminum and the other material shall be prevented as specified in those sections or by placing a compatible, nonporous isolator between the aluminum and the other material.
6.7.1 Steel
Steel surfaces to be placed in contact with uncoated aluminum shall be painted with a coating suitable for the service. Where very corrosive conditions are expected, additional protection can be obtained by applying a sealant that excludes moisture from the joint during service. Aluminized, hot-dip galvanized or electro-galvanized steel in contact with aluminum need not be painted. Stainless steel (300 series) in contact with aluminum need not be painted except in high chloride environments"
But the more I have dug into the subject it doesn't seem like just having the steel elements galvanized is enough precaution. This will be an indoor application, but large garage doors open it up to quite high levels of humidity at times.
I've also found the suggestion of painting any dissimilar contact surface with one coat of zinc chromate primer and then one heavy coat of aluminum pigmented asphalt paint.
If anyone has any insight they could share to the best path forward the better. Thanks in advace.