dwf_90
Mechanical
- Feb 3, 2021
- 3
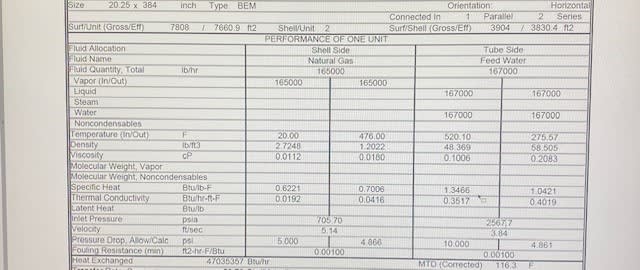
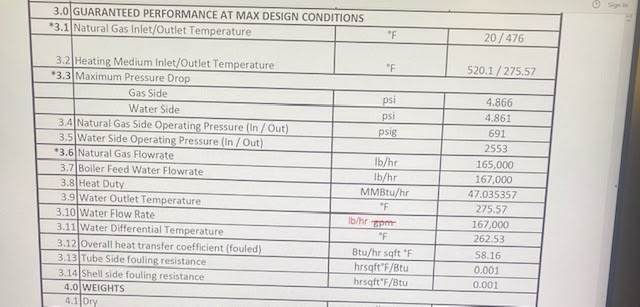
I am looking for help calculating the condensate temperature being sprayed to outside atmosphere via a leak. The source comes from a tube and shell heat exchanger using HP steam to heat natural gas. We have a leak on the steam side and management is asking to attempt to tight the flanges of the heat exchanger heads, while it is under pressure. Thankfully for safety reason this is no longer being considered I am curious of the temperature that the condensate will be if some one had attempted to work on this steam leak while it it spraying to the atmosphere. This isnt the first time this issue has came up with this heat exchanger, previously the gasket has been cut aprox 1" in width sometimes in a few different spots. Again not attempting to work on this equipment while under any amount of pressure or temperature im just curious so I can present some good data to our safety department and managers. Management wanted to reduce pressure from 1858 psi, 530 F to 500 psi, 452 F and 500 psi,385 F. i would like to know the calculation or how to calculate the condensate temperature when the exchanger is at 500 psi , 452F and 500 psi 385F? Thank in advance.