AdamPrince2
Automotive
- Dec 31, 2017
- 4
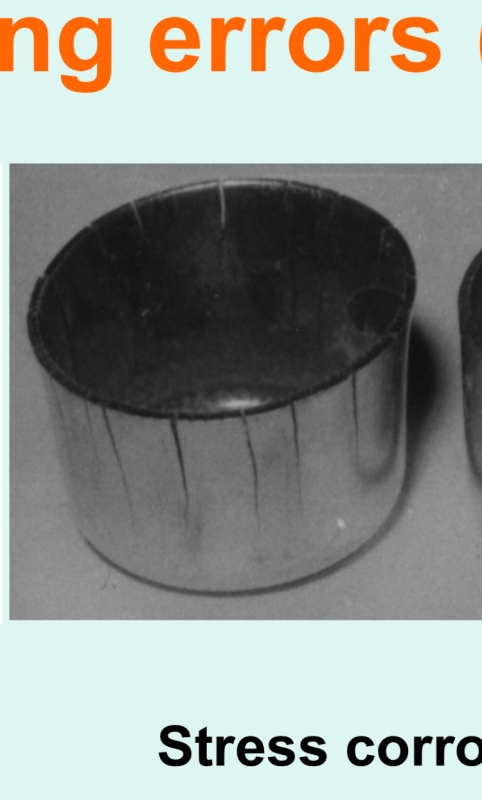
I saw a cooking pot that was made of stainless steel. It was used to cook potato soup. After cooking a restaurant owner decided to soak the pot in hot soapy water over night in the sink. The next day he found the pot literally cracked up like a banana peel! I have attached a photo. What failure mechanism would cause such a spectacular failure of a cooking pot?



![[hourglass] [hourglass] [hourglass]](/data/assets/smilies/hourglass.gif)