-
2
- #1
JoeFrickinFriday
Mechanical
You've probably seen this by now, but just in case, last fall Destin Sandlin uploaded a video about a pneumatic baseball cannon he and some friends built that can launch baseballs at well over 1,000 MPH. There's a 23-minute video in which they describe the design, constructions, and early tests of it:
Destin uploaded a companion video a few months later, in which he and his fellows fire baseballs through a variety of items through which you should not be able to fire a baseball:
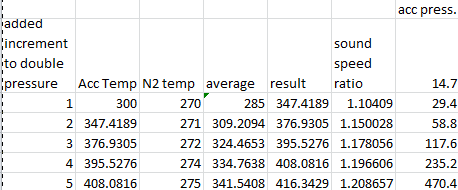
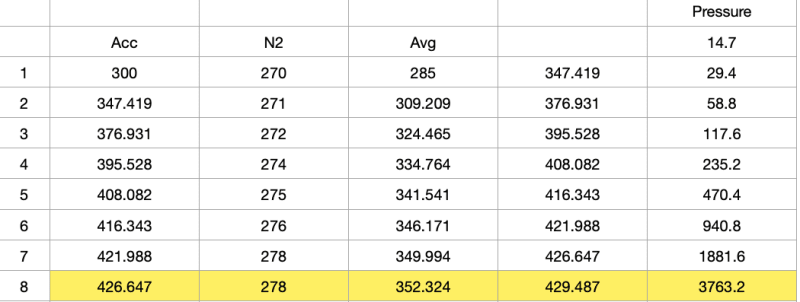
My question concerns the first video. at 2:48, we see a 2-D CAD printout showing a critical flow venturi in the barrel just upstream of where the baseball begins its journey. This made sense to me, as it seems like a CFV would be needed to accelerate the driving gas past sonic velocity. Without a CFV, ISTM the tank and barrel together would form a CFV with an exit-to-throat ratio of 1 (so I guess something akin to a critical flow orifice), making it impossible to go faster than the local sonic velocity. But a little while later at 3:30, we see a SolidWorks model that shows no CFV in the barrel. So what gives? I asked this question in the comment section, but got no response (no hurt feelings on my parts, he's got a lot of videos with a lot of comments and can't respond to all of them).
So I'm wondering what the collective here has to say. Is a CFV necessary for achieving supersonic muzzle velocity in a pneumatic cannon like this, or am I missing something about compressible flow?
Thanks...
Destin uploaded a companion video a few months later, in which he and his fellows fire baseballs through a variety of items through which you should not be able to fire a baseball:
My question concerns the first video. at 2:48, we see a 2-D CAD printout showing a critical flow venturi in the barrel just upstream of where the baseball begins its journey. This made sense to me, as it seems like a CFV would be needed to accelerate the driving gas past sonic velocity. Without a CFV, ISTM the tank and barrel together would form a CFV with an exit-to-throat ratio of 1 (so I guess something akin to a critical flow orifice), making it impossible to go faster than the local sonic velocity. But a little while later at 3:30, we see a SolidWorks model that shows no CFV in the barrel. So what gives? I asked this question in the comment section, but got no response (no hurt feelings on my parts, he's got a lot of videos with a lot of comments and can't respond to all of them).
So I'm wondering what the collective here has to say. Is a CFV necessary for achieving supersonic muzzle velocity in a pneumatic cannon like this, or am I missing something about compressible flow?
Thanks...