Miran Fernando
Mechanical
Hello,
New user here, excited to draw on knowledge of others.
I have a small box to contain a science apparatus. The science apparatus needs to be kept cool and has inlet and outlets for fluid tubes. Sorry for this question if it is silly: do I need to cool the inside of the box as well? If I am actively cooling the apparatus, aren't I also cooling the inside air?
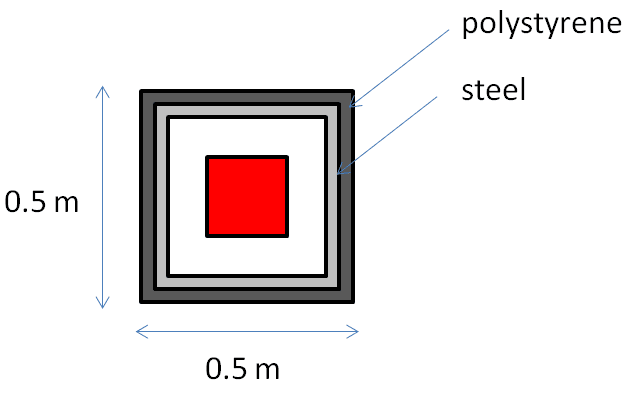
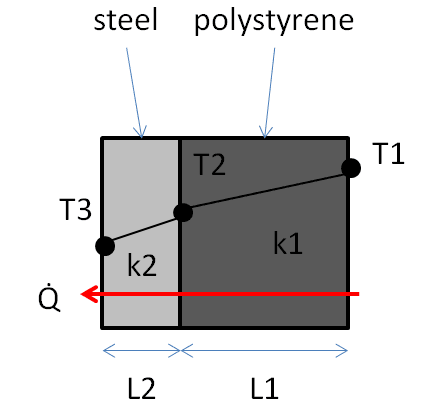
Some background. The box (which will be insulated) will be stored in a small shed outside that will be exposed to the ambient air. There are times of the day when the box may be in direct sunlight. The science apparatus will be cooled by a nearby chiller, so not too worried about it getting very warm. But I am unsure about the air inside the box. Doesn't the chiller need to "work harder" if the air begins warming up?
This is not my usual area of expertise and any guidance would be much appreciate.
Many thanks,
Miran
New user here, excited to draw on knowledge of others.
I have a small box to contain a science apparatus. The science apparatus needs to be kept cool and has inlet and outlets for fluid tubes. Sorry for this question if it is silly: do I need to cool the inside of the box as well? If I am actively cooling the apparatus, aren't I also cooling the inside air?
Some background. The box (which will be insulated) will be stored in a small shed outside that will be exposed to the ambient air. There are times of the day when the box may be in direct sunlight. The science apparatus will be cooled by a nearby chiller, so not too worried about it getting very warm. But I am unsure about the air inside the box. Doesn't the chiller need to "work harder" if the air begins warming up?
This is not my usual area of expertise and any guidance would be much appreciate.
Many thanks,
Miran