wright44
Civil/Environmental
- Jul 8, 2022
- 14
Hello,
Background:
I am working on a potable project for some folks who are looking to add an RO unit to their potable plant. The plant has an existing RO that was built and permitted some time ago for Arsenic removal. They are adding a new unit to increase throughput as demands are increasing. There are some fussy complications with the project, but essentially we are stepping in to help fill gaps and get it permitted, but to do so we need to validate the design.
The Issue:
Naturally, RO can produce some aggressive water and we anticipate this one is no different. Due to the current spotlight on Lead and Copper rule violations around the country, our regulator is very concerned about treatments that lead to aggressive water. The proposed RO unit utilizes a Calcite filter to attempt to raise pH and adjust the final alkalinity. The water sits in a storage tank open to atmosphere before final delivery, so I also have to assume there will be an equilibrium with air before distribution. The issue I am having is properly validating that the calcite filter is sufficient.
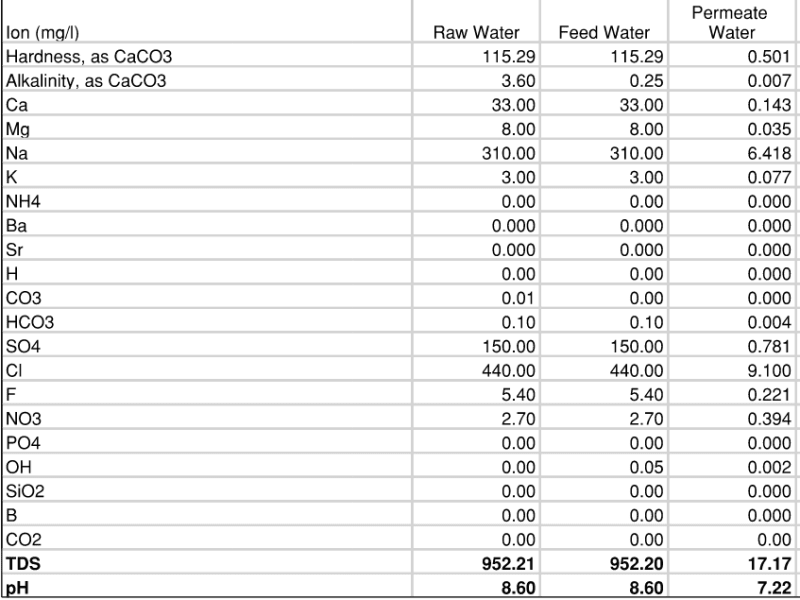
I have been using this spreadsheet to try and simulate various water conditions and the effect it may have on the finished water quality. Based on what we know, the permeate from the RO will have incredibly low alkalinity and calcium (nearly 0). The pH typically ranges from 7.0 to 7.4. Water temperature around 20 Celsius. Resulting Langelier Index is -7.27 and Calcium Carbonate Precipitation Potential (CCPP) is -12.6 aka VERY AGGRESSIVE.
From my calculations, it seems like no matter what dose of Calcite (as a function of bed depth) we use will lead to a neutral or non corrosive end point. Hoping some folks with experience on these things could help provide insight. I have seen recommendations to dose CO2, which I simulated and it does lead to a better end point. If that is the only solution, I will need to make recommendation but that will complicate things for the client and operator.
Data
INPUTS
Water Parameters
pre-Calcite pH: 7 to 7.4
pre-Calcite Alkalinity: 0.007 to 5 mg/L as CaCO3
pre-Calcite Calcium: 0.143 to 5 mg/L as Ca
Water Temperature: 20 Celsius
Calcite Filter
Bed Depth: 6 feet
Superficial Velocity: 8gpm/ft^2 (Notably higher than recommended 1 to 2 gpm/ft^2)
Finished Goals
pH: 8 to 8.5
Langelier Index: -0.3 to 0.3
CCPP: >0
Generally needs to be sufficient to avoid Lead & Copper corrosion.
Potential Solutions?
If calcite is not suitable for this water, will CO2 or some other means be sufficient?
References
I have been using the following references:
Limestone Contactors as a Corrosion Control Technology for Small Public Water Systems
Optimal Corrosion Control Treatment Evaluation Technical Recommendations for Primary Agencies and Public Water Systems
Any thoughts or recommendations are greatly appreciated.
Background:
I am working on a potable project for some folks who are looking to add an RO unit to their potable plant. The plant has an existing RO that was built and permitted some time ago for Arsenic removal. They are adding a new unit to increase throughput as demands are increasing. There are some fussy complications with the project, but essentially we are stepping in to help fill gaps and get it permitted, but to do so we need to validate the design.
The Issue:
Naturally, RO can produce some aggressive water and we anticipate this one is no different. Due to the current spotlight on Lead and Copper rule violations around the country, our regulator is very concerned about treatments that lead to aggressive water. The proposed RO unit utilizes a Calcite filter to attempt to raise pH and adjust the final alkalinity. The water sits in a storage tank open to atmosphere before final delivery, so I also have to assume there will be an equilibrium with air before distribution. The issue I am having is properly validating that the calcite filter is sufficient.
I have been using this spreadsheet to try and simulate various water conditions and the effect it may have on the finished water quality. Based on what we know, the permeate from the RO will have incredibly low alkalinity and calcium (nearly 0). The pH typically ranges from 7.0 to 7.4. Water temperature around 20 Celsius. Resulting Langelier Index is -7.27 and Calcium Carbonate Precipitation Potential (CCPP) is -12.6 aka VERY AGGRESSIVE.
From my calculations, it seems like no matter what dose of Calcite (as a function of bed depth) we use will lead to a neutral or non corrosive end point. Hoping some folks with experience on these things could help provide insight. I have seen recommendations to dose CO2, which I simulated and it does lead to a better end point. If that is the only solution, I will need to make recommendation but that will complicate things for the client and operator.
Data
INPUTS
Water Parameters
pre-Calcite pH: 7 to 7.4
pre-Calcite Alkalinity: 0.007 to 5 mg/L as CaCO3
pre-Calcite Calcium: 0.143 to 5 mg/L as Ca
Water Temperature: 20 Celsius
Calcite Filter
Bed Depth: 6 feet
Superficial Velocity: 8gpm/ft^2 (Notably higher than recommended 1 to 2 gpm/ft^2)
Finished Goals
pH: 8 to 8.5
Langelier Index: -0.3 to 0.3
CCPP: >0
Generally needs to be sufficient to avoid Lead & Copper corrosion.
Potential Solutions?
If calcite is not suitable for this water, will CO2 or some other means be sufficient?
References
I have been using the following references:
Limestone Contactors as a Corrosion Control Technology for Small Public Water Systems
Optimal Corrosion Control Treatment Evaluation Technical Recommendations for Primary Agencies and Public Water Systems
Any thoughts or recommendations are greatly appreciated.