timfinnegan
Mechanical
As the title suggests, can the JT effect be used to explain why the pressure drops across a thermal expansion valve for a typical refrigeration system (e.g. air conditioner or heat pump)?
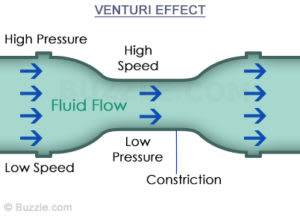
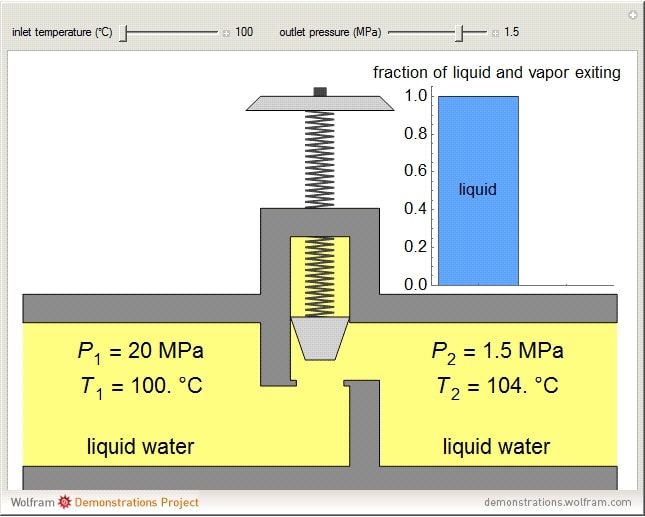
From what I understand, the liquid refrigerant dropping in pressure as it goes through a narrower passage can be more or less approximated by Bernoulli's equation as it speeds up, however, by the time the fluid exits the TXV, Bernoulli's equation no longer applies because the incompressibility assumption no longer holds true.
The problem is that, at least from what I've been reading online, the JT effect doesn't seem to account for phase change?
Also, does the fluid become a saturated mixture while it's in the TXV or right after it leaves the TXV?
And as a final question, for the venturi effect, why does the pressure drop in the narrow passage and then recover (as per Bernoulli's equation) yet the JT effect does not have this recovery effect? The pressure seems to drop permanently even after leaving the narrow passage into a larger space. What is the physics underlying these two different effects?
From what I understand, the liquid refrigerant dropping in pressure as it goes through a narrower passage can be more or less approximated by Bernoulli's equation as it speeds up, however, by the time the fluid exits the TXV, Bernoulli's equation no longer applies because the incompressibility assumption no longer holds true.
The problem is that, at least from what I've been reading online, the JT effect doesn't seem to account for phase change?
Also, does the fluid become a saturated mixture while it's in the TXV or right after it leaves the TXV?
And as a final question, for the venturi effect, why does the pressure drop in the narrow passage and then recover (as per Bernoulli's equation) yet the JT effect does not have this recovery effect? The pressure seems to drop permanently even after leaving the narrow passage into a larger space. What is the physics underlying these two different effects?