Hi,
I have uploaded two images. One is a table of Low Pressure Air Properties. The other is a typical psychrometric chart.
If you look up the enthalpy of air at 60F (520 R) in the low pressure air table, you get an enthalpy of 127.27 btu/lbm.
If you look up the enthalpy of air with zero humidity (dry air) in the psychrometric chart at 60F, you get an enthalpy of roughly 13 btu/lbm.
I know I am making some basic mistake here. Can anyone spot why these values do not match or what mistake I am making?
Thanks

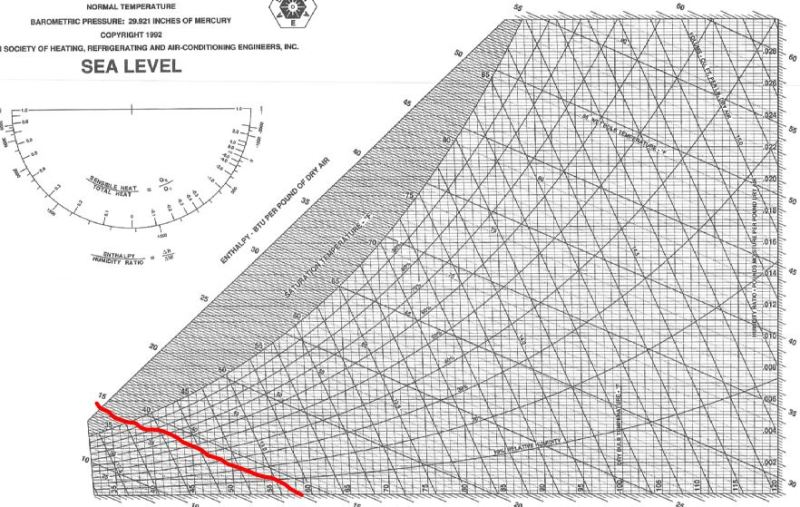
I have uploaded two images. One is a table of Low Pressure Air Properties. The other is a typical psychrometric chart.
If you look up the enthalpy of air at 60F (520 R) in the low pressure air table, you get an enthalpy of 127.27 btu/lbm.
If you look up the enthalpy of air with zero humidity (dry air) in the psychrometric chart at 60F, you get an enthalpy of roughly 13 btu/lbm.
I know I am making some basic mistake here. Can anyone spot why these values do not match or what mistake I am making?
Thanks


