-
1
- #1
Peter C
Chemical
- Nov 4, 2022
- 16
Hello,
Very commonly people we hear that a tank where column pressure is not big enough to overcome Patm will not empty by itself. In my opinion, most of those who say that forget that once the tank was open to be fulled and, therefore, we must add 1atm pressure to the fluid at any point.
So lets consider the following case: 1) a tank of height H is completly empty, 2) fluid (water in this case) is being pumped in through a valve in the bottom side while air is comming out at the top side, 3) once the tank is completly full, both valves are closed and 4) the valve at the bottom is opened.
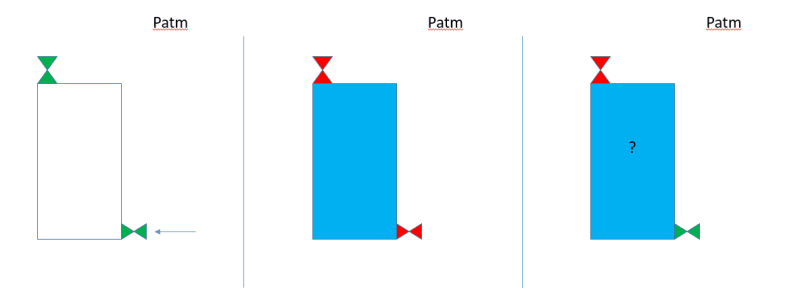
When the valve at the bottom is opened, what happens with the fluid inside? Will some of it come out, creating a "void' in the top of the tank (p = pvapor of the fluid)? NOTHING will come out? It will completly empty?
For now, lets ignore that air bubbles may come in from the bottom.
Can you answer using Bernoulli?
Regards
Very commonly people we hear that a tank where column pressure is not big enough to overcome Patm will not empty by itself. In my opinion, most of those who say that forget that once the tank was open to be fulled and, therefore, we must add 1atm pressure to the fluid at any point.
So lets consider the following case: 1) a tank of height H is completly empty, 2) fluid (water in this case) is being pumped in through a valve in the bottom side while air is comming out at the top side, 3) once the tank is completly full, both valves are closed and 4) the valve at the bottom is opened.

When the valve at the bottom is opened, what happens with the fluid inside? Will some of it come out, creating a "void' in the top of the tank (p = pvapor of the fluid)? NOTHING will come out? It will completly empty?
For now, lets ignore that air bubbles may come in from the bottom.
Can you answer using Bernoulli?
Regards