NalaCap0ne
Structural
Hi All,
I've run into a bit of an odd issue (for me anyways) on site the other day and was hoping to get some helpful input. For info, this site is in London, UK.
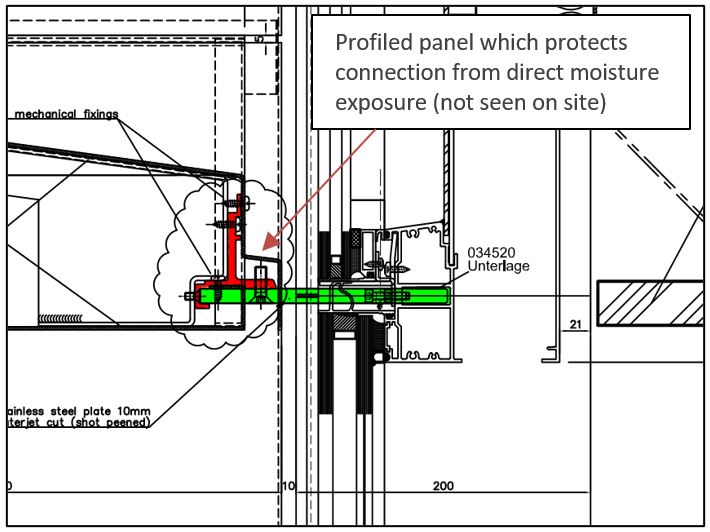
There is a 10mm THK stainless steel bracket (shown in green in the sketch, grade unknown) which is fixed to an aluminium profile that sits inside a brise-soleil panel. The fixing is not protected by the panel as is shown in the drawing, the connection is open to the elements.
As you can see in the photographs I have provided (one of the bracket, one of the aluminium profile), when the brise-soleil panels were removed, a later of aluminium oxide (I am assuming) has formed on the stainless steel, in the area where the two metals were in contact. What I think has happened based on the information I have gathered over the last few days is that the two dissimilar metals in contact and exposed to rainwater has created a galvanic reaction, causing the aluminium to corrode. The galvanic series chart which I am referencing shows stainless steel twice...once in the passive state and once in the active state. Again, what I am assumign has happened is that the stainless and aluminium oxide layers were damged during installation, and since no oxygen can get to the surfaces of these materials, corrosion began to occur. This coul dbe completely wrong...its just my thinking at the moment.
Can anyone provide me with any input on why this is occuring, when a stainless steel is passive or active, and what sort of long term effects can be expected?
Thanks in advance.


I've run into a bit of an odd issue (for me anyways) on site the other day and was hoping to get some helpful input. For info, this site is in London, UK.
There is a 10mm THK stainless steel bracket (shown in green in the sketch, grade unknown) which is fixed to an aluminium profile that sits inside a brise-soleil panel. The fixing is not protected by the panel as is shown in the drawing, the connection is open to the elements.
As you can see in the photographs I have provided (one of the bracket, one of the aluminium profile), when the brise-soleil panels were removed, a later of aluminium oxide (I am assuming) has formed on the stainless steel, in the area where the two metals were in contact. What I think has happened based on the information I have gathered over the last few days is that the two dissimilar metals in contact and exposed to rainwater has created a galvanic reaction, causing the aluminium to corrode. The galvanic series chart which I am referencing shows stainless steel twice...once in the passive state and once in the active state. Again, what I am assumign has happened is that the stainless and aluminium oxide layers were damged during installation, and since no oxygen can get to the surfaces of these materials, corrosion began to occur. This coul dbe completely wrong...its just my thinking at the moment.
Can anyone provide me with any input on why this is occuring, when a stainless steel is passive or active, and what sort of long term effects can be expected?
Thanks in advance.



