drogones12
Civil/Environmental
Hi All,
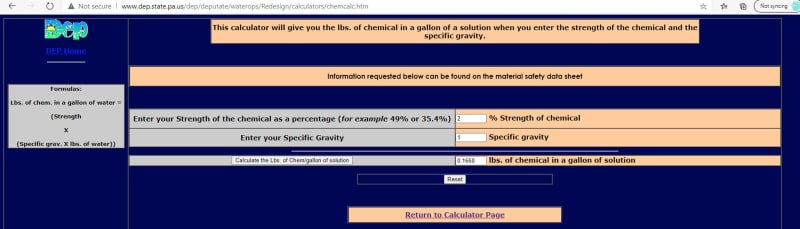
I am adding a KMnO4 chemical feed to my filtration compact package unit for the iron and manganese removal. I have a 50 gallon mixing day-tank and will be using powder KMnO4 for the solute to get 2% strength solution. The CAIROX potassium permanganate powder is 97% guaranteed with 2.703 g/cm^3 specific gravity. I've used the PADEP chemical calculator and got 0.451 lb/gallon solution as the result. So I would use 0.451 lb/gallon X 50 gallon = 22.55lb for 1 batch of my solution. Is this correct? Is there a formula to calculate the amount of KMnO4 that I need to be using? Thank you for your input.
thread164-432736
I am adding a KMnO4 chemical feed to my filtration compact package unit for the iron and manganese removal. I have a 50 gallon mixing day-tank and will be using powder KMnO4 for the solute to get 2% strength solution. The CAIROX potassium permanganate powder is 97% guaranteed with 2.703 g/cm^3 specific gravity. I've used the PADEP chemical calculator and got 0.451 lb/gallon solution as the result. So I would use 0.451 lb/gallon X 50 gallon = 22.55lb for 1 batch of my solution. Is this correct? Is there a formula to calculate the amount of KMnO4 that I need to be using? Thank you for your input.
thread164-432736