gotothesky
Mechanical
I am an exchanger thermal design engineer.
I wonder how to do design of stripping column.
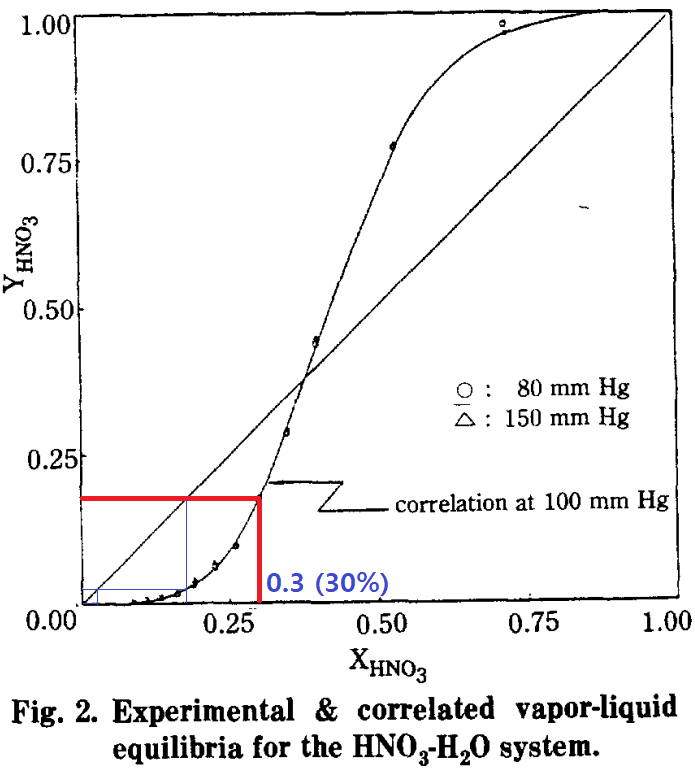
This stripping column is for removing Nitric and Sulfuric acid from water.
From Top of stripping column, Pure water comes out.
This kind of equipment is normally designed with process simulation software such like Aspen?
Or Is there any simple software?
Thank you in advance.
NOTE : The water from top of stripping column should be pure without any acid!
and Reflux can be done for stripping column.
I wonder how to do design of stripping column.
This stripping column is for removing Nitric and Sulfuric acid from water.
From Top of stripping column, Pure water comes out.
This kind of equipment is normally designed with process simulation software such like Aspen?
Or Is there any simple software?
Thank you in advance.
NOTE : The water from top of stripping column should be pure without any acid!
and Reflux can be done for stripping column.