-
1
- #1
theeyesofahunter
Civil/Environmental
Context:
I want to cool my house by heat piping between the ground/basement space to living space of the floor above.
There is 5m vertically between ceiling of the first floor and the floor of the basement.
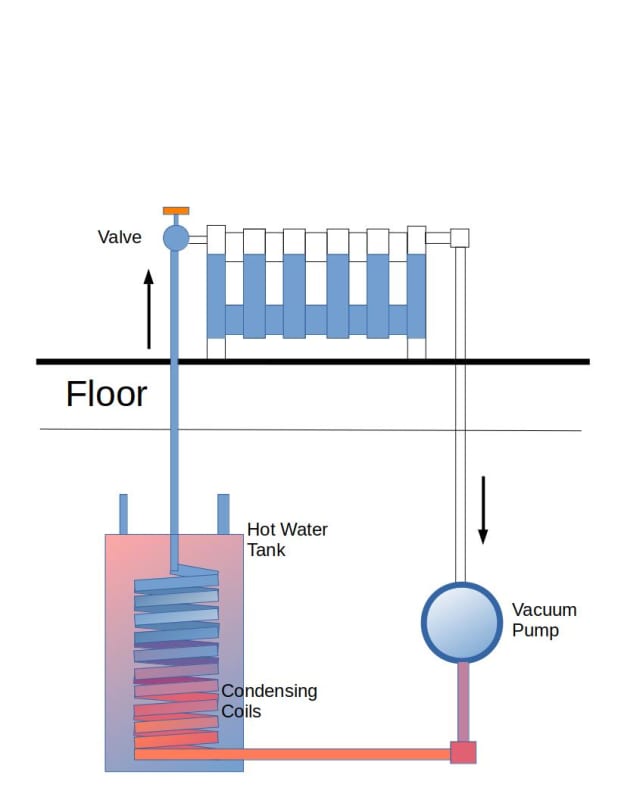
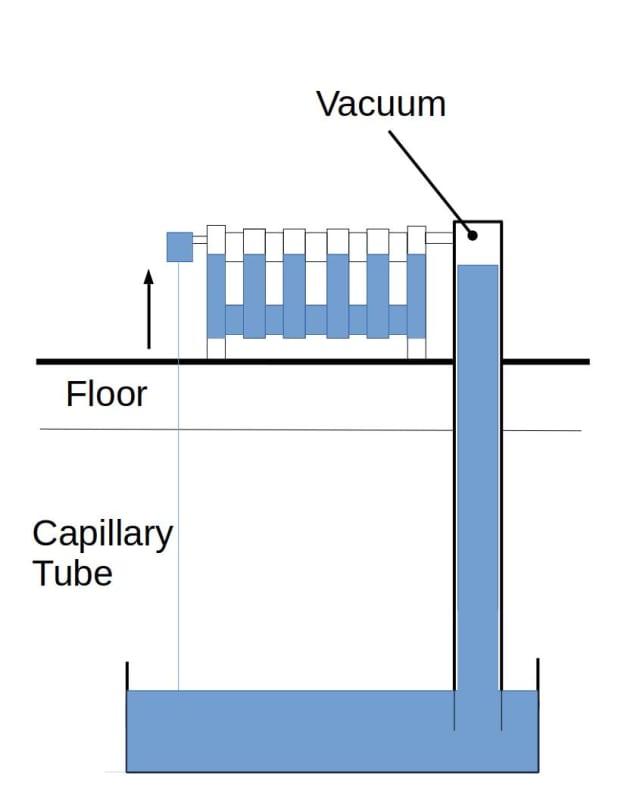
My plan is to make a loop 5m long (vertically) filled with water, up to, 4m and vacuum out the air with a pump (and guage), so that water will boil at 20c.
The heat input will be a heat sink placed vertically on the pipe/wall (with 4m being the mid point of the heat sink)
and a second heat sink in the basement , likely on the floor.
Reading suggests that applying heat at the top usually results in a lack fluid to at that point and hence no cycling of the working fluid.
As a apposed to applying heat at the bottom where the working fluid boils and cycles through evaporation/condensation as its fed by gravity back.
My question is: would filling the fluid almost to the top (i.e. the heat input) cycle?
(and in a useful way?)
I want to cool my house by heat piping between the ground/basement space to living space of the floor above.
There is 5m vertically between ceiling of the first floor and the floor of the basement.
My plan is to make a loop 5m long (vertically) filled with water, up to, 4m and vacuum out the air with a pump (and guage), so that water will boil at 20c.
The heat input will be a heat sink placed vertically on the pipe/wall (with 4m being the mid point of the heat sink)
and a second heat sink in the basement , likely on the floor.
Reading suggests that applying heat at the top usually results in a lack fluid to at that point and hence no cycling of the working fluid.
As a apposed to applying heat at the bottom where the working fluid boils and cycles through evaporation/condensation as its fed by gravity back.
My question is: would filling the fluid almost to the top (i.e. the heat input) cycle?
(and in a useful way?)