Now we're getting somewhere.
What I don't think any of us understand at the moment is how do you cool the returning gaseous CO2?
Somewhere if you want a substance to stay below a certain temperature then you need to have a cooling mechanism. This can be boiling off the liquid or some mechanical means such as a chiller loop.
Can you actually sketch out your complete loop system with pressures and temperatures where known. A consultant will ask for this in the first instance or charge you to create one.
Some confusing things - you say you tried to get drawings from a manufacturer but it was too old - but in the OP it said you had just installed it??
What is the pressure and temperature in the tank in the tank??
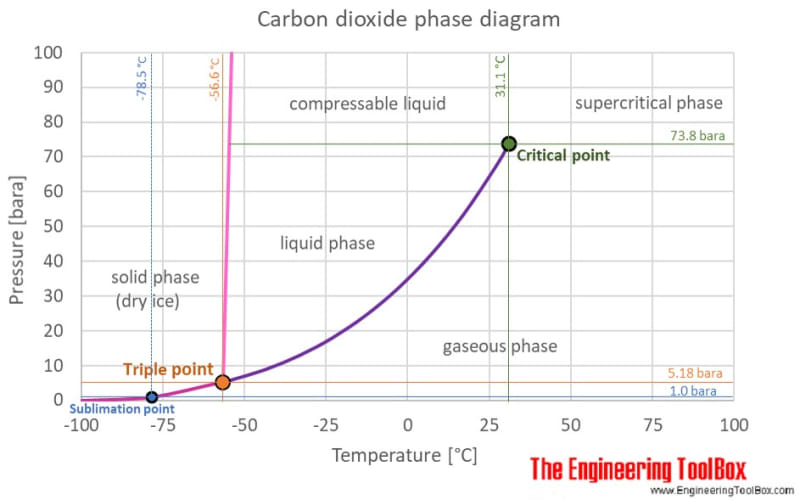
When you know that you can find out where on the phase diagram you are.
You state the pressure at the discharge of the pump as 285 but not it's inlet pressure ( though you state storage pressure is 285 so what is the pump doing??)
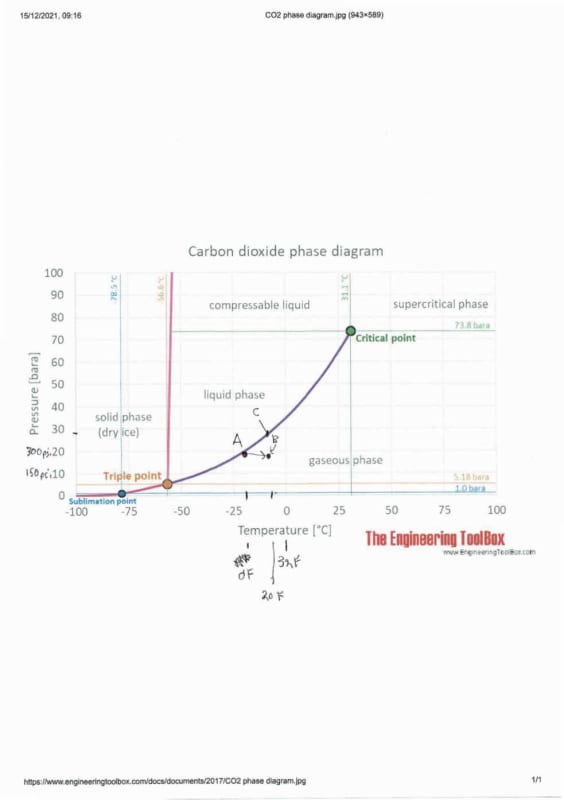
To understand CO2 you need to use the phase diagram I posted above and annotated below.
It sounds to me that in your storage tank (285 psi @ 0F) you may just be at point A where there is liquid CO2 in the tank. But it might be a tank of gas as this is right on the line. Even if it's a liquid if you maintain the same pressure ( how do you get it to flow?) and the temperature increases as you go along your line, then the liquid CO2 will boil as it moves right on the graph but at the same or similar pressure. Therefore you will get gas at your mould at point B.
Then somehow it returns to the tank as a gas. There needs then to be some mechanism to cool it back down to 0F to liquify it.
Long and short of it is that if you want to get liquid CO2 at 285 psi to the mould the temperature needs to be 0F or lower. Or if the temperature is 20F then you need to arrive at point C - about 400 psi.
Does this make sense?
Remember - More details = better answers
Also: If you get a response it's polite to respond to it.