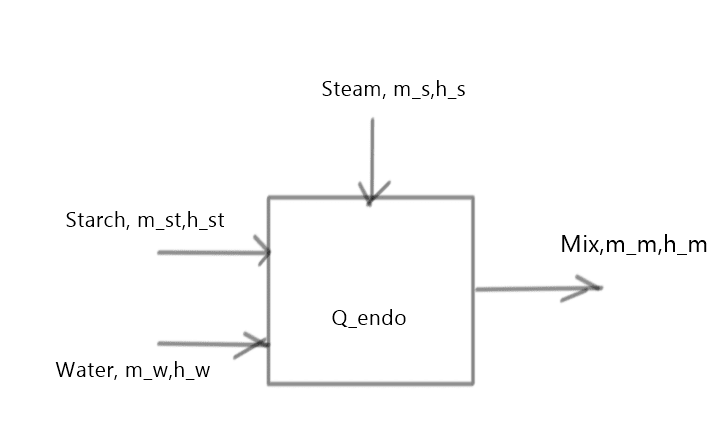
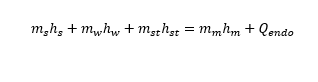Hi,
I have all data on mixing vapor, starch and water (temperatures, pressure) and when I make a balance:
m_vapor_in*h_vapor_in+m_water*cp_water*T_water=(m_water_in+m_vapor_in)*cp_water*T_mix_measured+m_starch*
cp_starch_new*T_mix_measured+E_endo)
I get a result, that expression on left is 1,9x bigger than on the right side. I took into account that cp,new (starch) is temperature dependent.
Regarding new mixing temperature (95˙C) I understand that I get some heat loss, but not almost by factor 2? I neglected reactor mass. Mass of vapor is determined by measuring mixture level before and after measurment. (V=3,14*D^2/4*(h2-h1)...m_water=V*ro_water=m_vapor_in
thanks
I have all data on mixing vapor, starch and water (temperatures, pressure) and when I make a balance:
m_vapor_in*h_vapor_in+m_water*cp_water*T_water=(m_water_in+m_vapor_in)*cp_water*T_mix_measured+m_starch*
cp_starch_new*T_mix_measured+E_endo)
I get a result, that expression on left is 1,9x bigger than on the right side. I took into account that cp,new (starch) is temperature dependent.
Regarding new mixing temperature (95˙C) I understand that I get some heat loss, but not almost by factor 2? I neglected reactor mass. Mass of vapor is determined by measuring mixture level before and after measurment. (V=3,14*D^2/4*(h2-h1)...m_water=V*ro_water=m_vapor_in
thanks