Furious.George
Computer
- Mar 23, 2019
- 13
I have some software I wrote that I'd like to apply to heat moving/storage, for fun.
To that end, I've been studying the vapor compression cycle. This is complicated by the fact that I only have a general understanding of thermodynamics. The purpose of this post is put in writing what I think are reasonable expectations, in order to see if they are reasonable.
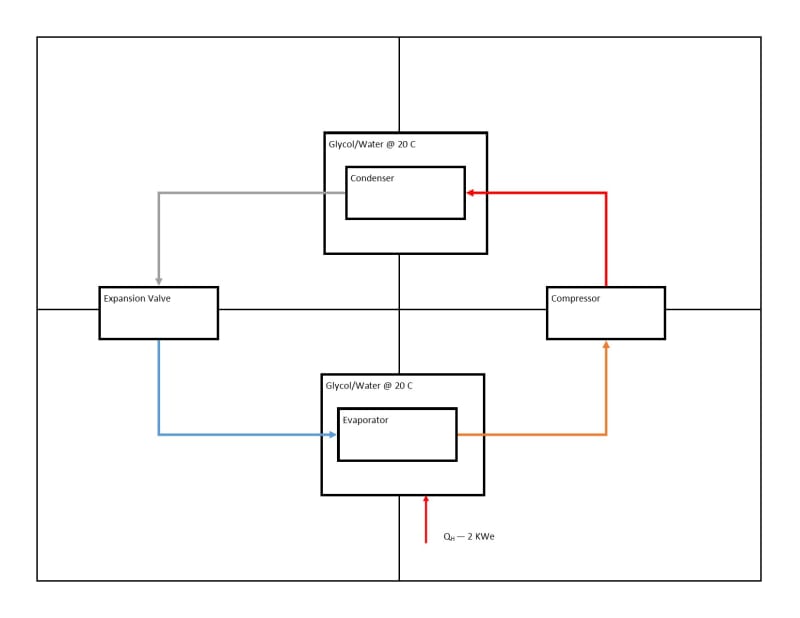
I want to extract heat from vessel with water/glycol @ 90 C, and put it in a vessel that is initially at 20 C.
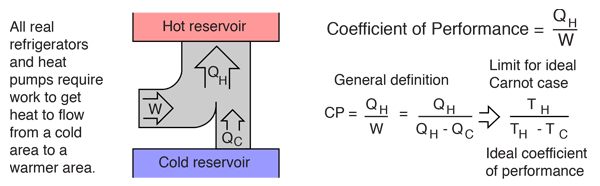
If my understanding of the cycle is correct, which it probably is not, taking thermal energy from one side will increase the energy on the other by a similar amount, less the energy lost in transport. Efficiency will decrease as the destination vessel gets warmer. Once the condenser in destination vessel is at a similar temperature to the liquid in the same vessel, then heat transfer will stop. Depending on efficiency, this can be somewhere between 7 and 30 C of delta between evaporator and condenser.
Putting this all together, I should expect to reach an equilibrium where the heat loss from the entire system is equal to the input for my source water/glycol, and the delta between the condenser and evaporator is around 20 C. That might mean the source is at 70 C now, and the destination is at 90 C.
Does that sound right?
To that end, I've been studying the vapor compression cycle. This is complicated by the fact that I only have a general understanding of thermodynamics. The purpose of this post is put in writing what I think are reasonable expectations, in order to see if they are reasonable.
I want to extract heat from vessel with water/glycol @ 90 C, and put it in a vessel that is initially at 20 C.
If my understanding of the cycle is correct, which it probably is not, taking thermal energy from one side will increase the energy on the other by a similar amount, less the energy lost in transport. Efficiency will decrease as the destination vessel gets warmer. Once the condenser in destination vessel is at a similar temperature to the liquid in the same vessel, then heat transfer will stop. Depending on efficiency, this can be somewhere between 7 and 30 C of delta between evaporator and condenser.
Putting this all together, I should expect to reach an equilibrium where the heat loss from the entire system is equal to the input for my source water/glycol, and the delta between the condenser and evaporator is around 20 C. That might mean the source is at 70 C now, and the destination is at 90 C.
Does that sound right?