takiyasamsama
Chemical
I need to find the properties of a stream which is in two-phase. I'm only given separate properties i.e. liquid and vapor properties. I need to find the enthalpy for the two-phase mixture from the liquid and vapor enthalpy. See the table below:
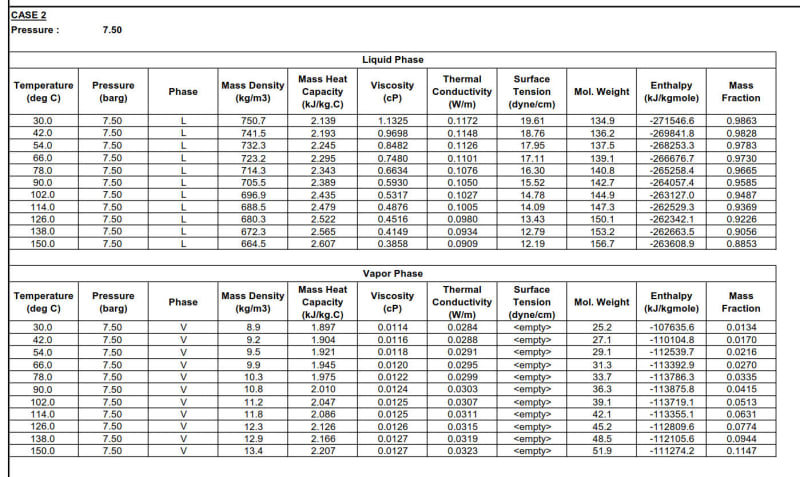
I would really like to know how do I find mixture properties i.e. density, thermal conductivity, specific heat, viscosity & enthalpy

I would really like to know how do I find mixture properties i.e. density, thermal conductivity, specific heat, viscosity & enthalpy
