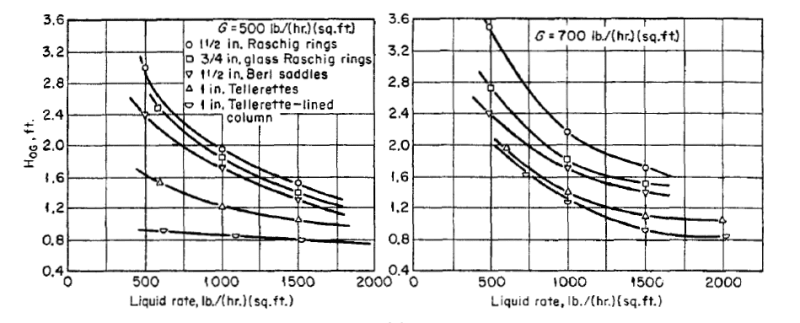
Hi Guys,
I need your help on absorption HTU. Please refer to attached. It's related to base system HTU from KGa curve. How do I get HTU using formula HTU= G/ Kga?
Below is the test conditions:
Bed height 3.05m
Liquid concentration: 4% NaOH
Conversion to carbonate:25%
Inlet CO2 concentration: 1%
Gas rate: 900 lb/hr ft3 atm, divide by air density 29 lb/lbmol= 31 lbmol/hr ft3 m
Pressure: 1 am
Assumptions:
1) This is chemical absorption so consider gas phrase only.
2) Assume at liquid rate 8 gpm/ft2 using Saddle #1. The Kga value is ~ 3 lbmol/hr ft3 atm
3) Using HTU= G/Kga= 31/3= 10.3 ft!
4) This HTU value is very high and not reasonable.
Thanks for attention and please help !
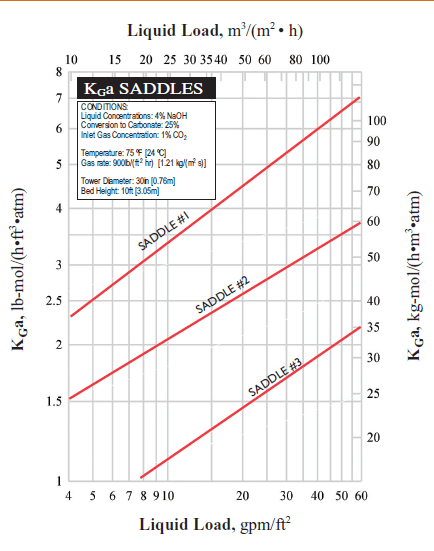
I need your help on absorption HTU. Please refer to attached. It's related to base system HTU from KGa curve. How do I get HTU using formula HTU= G/ Kga?
Below is the test conditions:
Bed height 3.05m
Liquid concentration: 4% NaOH
Conversion to carbonate:25%
Inlet CO2 concentration: 1%
Gas rate: 900 lb/hr ft3 atm, divide by air density 29 lb/lbmol= 31 lbmol/hr ft3 m
Pressure: 1 am
Assumptions:
1) This is chemical absorption so consider gas phrase only.
2) Assume at liquid rate 8 gpm/ft2 using Saddle #1. The Kga value is ~ 3 lbmol/hr ft3 atm
3) Using HTU= G/Kga= 31/3= 10.3 ft!
4) This HTU value is very high and not reasonable.
Thanks for attention and please help !