TomaszKruk
Civil/Environmental
- Oct 2, 2019
- 33
hello,
kinda newbish questions, but the specific answers are hard to come by from what I could google. Hope someone will take time to answer them.
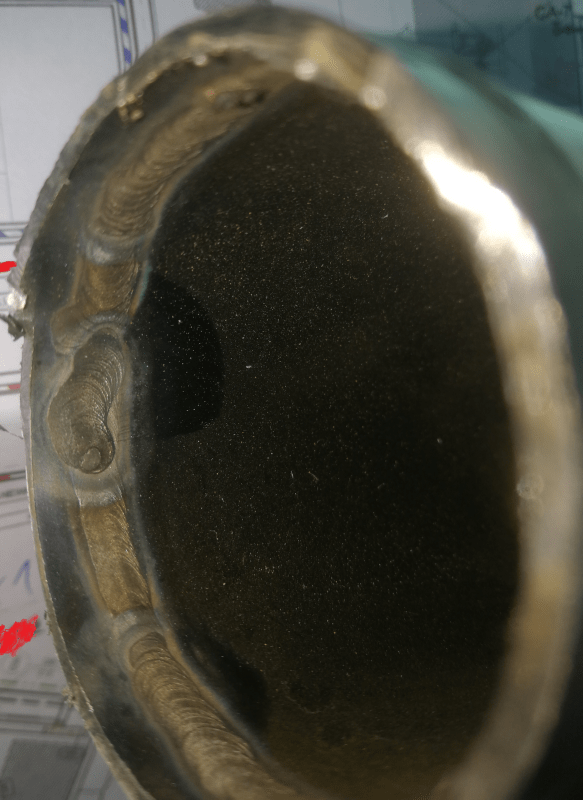
1) I'm currently on a job, when we are to modify an existing demi water installation. Having removed some pipes I noticed signs of oxidization on welds (from residual oxygen, nothing horrible - light yellow color). The installation was supposedly passivated during comission (way, way back). I was wondering - from my knowledge proper passivation includes washing the pipe with acid, and would mean that the discoloration of welds would be removed in the process. Could it have been that the pipes were not passivated at all? And still did not corrode badly? We plan to passivate the thing and our sub-contractor's brochures state that passivation should include cleaning the pipe with acid, and would lead to clean, "natural" stainless steel surface even on welds (it wouldn't polish the welds obviously - just remove any discoloration).
2) I read conflicting info on self passivation of stainless steel - would stainsless steel self passivate on weld surface? Found info both ways - that it would, or that the chromium layer would not rebuild on its own on the weld surface.
3) Could stainless steel self passivate in your typical demi water circuit? I don't know the exact specs of the installation, so it's hard to determine if the water contains sufficient amounts of oxygen.
4) From what I've managed to gather by taking a sneak peak at their storage room they use stuff containing blach (chlorium) to desinfect the pipes. Shouldn't that mess with unpassivated welds? Would the self passivation on welds be sufficient to protect the pipe from periodic contact with solutions of chlorium?
5) I reat somewhere that corrosion products of stainless steel would not readily contaminate the water unless the corrosion happened due to contact with carbon steel. Is this right?
I'm new to this whole corrosion thing and find it fascinating so far. Never thought there was so much to it till I got to see it for myself.
All in all - I'm wondering if we're looking at installation done right, or that corrosion in this case would be so slow that it doesn't show yet. I was led to believe that passivation was super crucial, and should be done immidately after welding. Now I'm wondering.
Cheers and thanks for any info,
kinda newbish questions, but the specific answers are hard to come by from what I could google. Hope someone will take time to answer them.
1) I'm currently on a job, when we are to modify an existing demi water installation. Having removed some pipes I noticed signs of oxidization on welds (from residual oxygen, nothing horrible - light yellow color). The installation was supposedly passivated during comission (way, way back). I was wondering - from my knowledge proper passivation includes washing the pipe with acid, and would mean that the discoloration of welds would be removed in the process. Could it have been that the pipes were not passivated at all? And still did not corrode badly? We plan to passivate the thing and our sub-contractor's brochures state that passivation should include cleaning the pipe with acid, and would lead to clean, "natural" stainless steel surface even on welds (it wouldn't polish the welds obviously - just remove any discoloration).
2) I read conflicting info on self passivation of stainless steel - would stainsless steel self passivate on weld surface? Found info both ways - that it would, or that the chromium layer would not rebuild on its own on the weld surface.
3) Could stainless steel self passivate in your typical demi water circuit? I don't know the exact specs of the installation, so it's hard to determine if the water contains sufficient amounts of oxygen.
4) From what I've managed to gather by taking a sneak peak at their storage room they use stuff containing blach (chlorium) to desinfect the pipes. Shouldn't that mess with unpassivated welds? Would the self passivation on welds be sufficient to protect the pipe from periodic contact with solutions of chlorium?
5) I reat somewhere that corrosion products of stainless steel would not readily contaminate the water unless the corrosion happened due to contact with carbon steel. Is this right?
I'm new to this whole corrosion thing and find it fascinating so far. Never thought there was so much to it till I got to see it for myself.
All in all - I'm wondering if we're looking at installation done right, or that corrosion in this case would be so slow that it doesn't show yet. I was led to believe that passivation was super crucial, and should be done immidately after welding. Now I'm wondering.
Cheers and thanks for any info,