Hi All
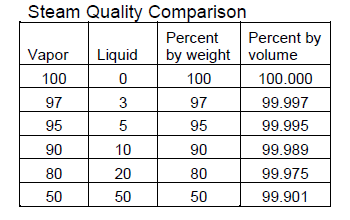
After conducted a throttling experiment with water and noting the results. I would like to verify if it they make sense. What I noticed as that when the pressure inside the sealed vessel increased from say 1 to 7 bar in 1 bar increments the quality factor of the steam remained fairly constant. It was around 0.96 or 96% and when it decreased from 7 bar to 1 bar the quality factor also remained fairly constant but was slightly higher around 0.97. Any Ideads
After conducted a throttling experiment with water and noting the results. I would like to verify if it they make sense. What I noticed as that when the pressure inside the sealed vessel increased from say 1 to 7 bar in 1 bar increments the quality factor of the steam remained fairly constant. It was around 0.96 or 96% and when it decreased from 7 bar to 1 bar the quality factor also remained fairly constant but was slightly higher around 0.97. Any Ideads