Willem19
Civil/Environmental
- Dec 13, 2022
- 10
Dear People,
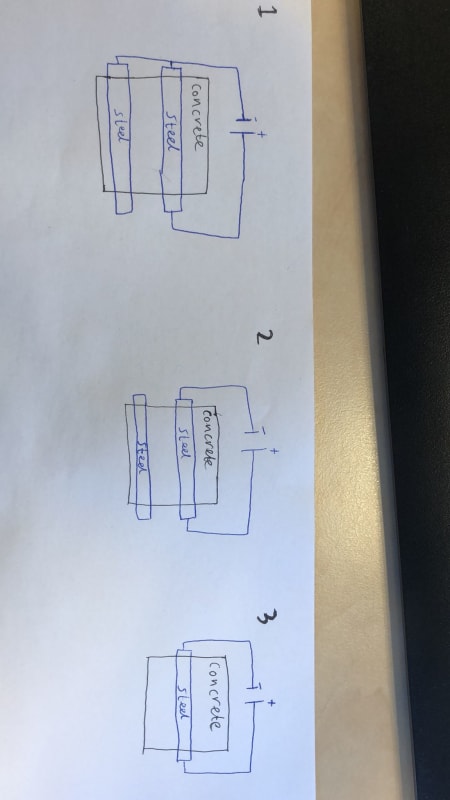
I am working on a project on which there is a possibility that stray current will occur. I know what stray current corrosion is in general, but I don't know why and when it occurs. Therefore I've made 3 setups which I may test to see if i can make a representation of my project. In my project a leakage current will flow directly on to reinforced concrete, and the current will flow away through lightning rods connected to the reinforced concrete. The lightning rods are made up of 1 ohm and are therefore not drawen in the setups. I am wondering if somebody knows beforehand in which of my 3 experimental setups corrosion will occure, and why. And if my setups are correct.
I am looking forward to a respone.
Kind regards,
Willem
I am working on a project on which there is a possibility that stray current will occur. I know what stray current corrosion is in general, but I don't know why and when it occurs. Therefore I've made 3 setups which I may test to see if i can make a representation of my project. In my project a leakage current will flow directly on to reinforced concrete, and the current will flow away through lightning rods connected to the reinforced concrete. The lightning rods are made up of 1 ohm and are therefore not drawen in the setups. I am wondering if somebody knows beforehand in which of my 3 experimental setups corrosion will occure, and why. And if my setups are correct.
I am looking forward to a respone.

Kind regards,
Willem
