Faiz iqbal
Chemical
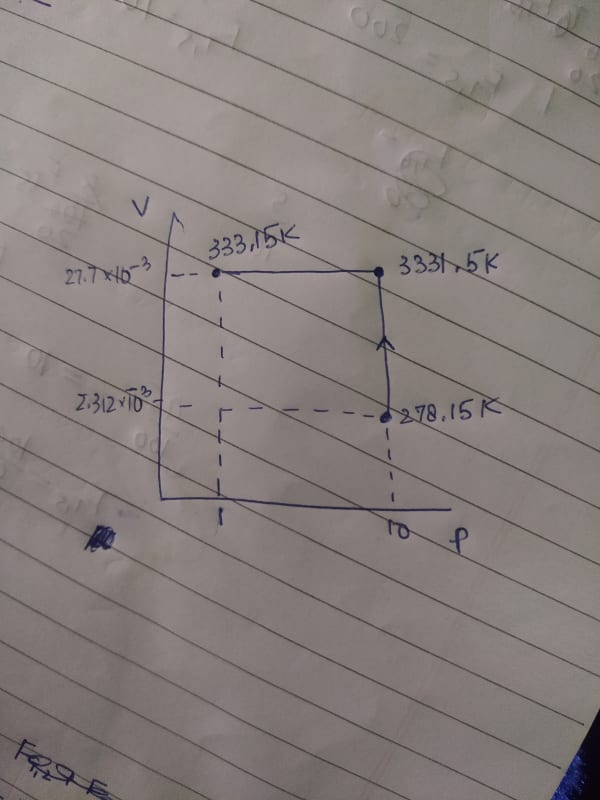
Cv = 20.785
Cp = 29.100
Can you please calculate the enthalpy change and internal energy change for the process. In my book the same problem is done with the other path. The problem is the final values just don't match. Please help.
Cp = 29.100
Can you please calculate the enthalpy change and internal energy change for the process. In my book the same problem is done with the other path. The problem is the final values just don't match. Please help.