MartinLe
Civil/Environmental
- Oct 12, 2012
- 394
"Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration (relative to that of the feed solution), is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes." (wikipedia)
In a second step, the diluted draw solution is pumped through RO membranes, leading to desalinated water and a thickened draw solution to be recycled. The stated benefit is that the FO membrane faces far less scaling than in an RO process.
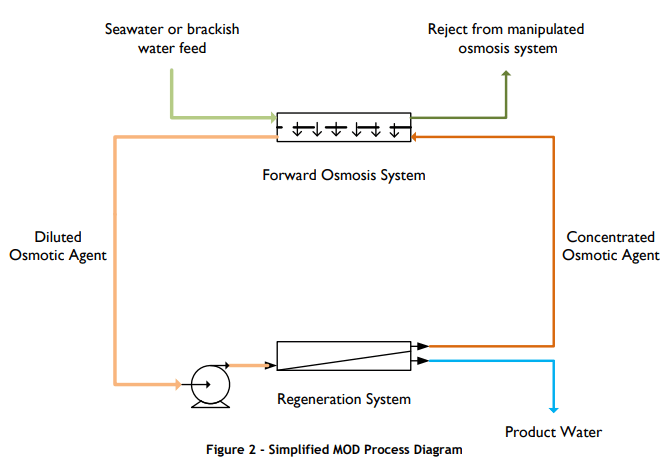
Now the electrolyte used as draw solution can be optimized so that no scaling on the RO membranes occurs. Since the manufactuers of these plants don't publish the composition of the draw solutions, I presume a lot of research went into that direction.
However, I have conceptual question on why FO is advantageuos at all: when water diffues through a membrane, the concentration of the solutes is higher near the membrane and this ultimately leads to scaling if hardness builders etc. are present. It is stated that this occurs far less when the diffusion is driven by osmotic pressure instead of pressure from a pump. Why is that so?
In a second step, the diluted draw solution is pumped through RO membranes, leading to desalinated water and a thickened draw solution to be recycled. The stated benefit is that the FO membrane faces far less scaling than in an RO process.

Now the electrolyte used as draw solution can be optimized so that no scaling on the RO membranes occurs. Since the manufactuers of these plants don't publish the composition of the draw solutions, I presume a lot of research went into that direction.
However, I have conceptual question on why FO is advantageuos at all: when water diffues through a membrane, the concentration of the solutes is higher near the membrane and this ultimately leads to scaling if hardness builders etc. are present. It is stated that this occurs far less when the diffusion is driven by osmotic pressure instead of pressure from a pump. Why is that so?
