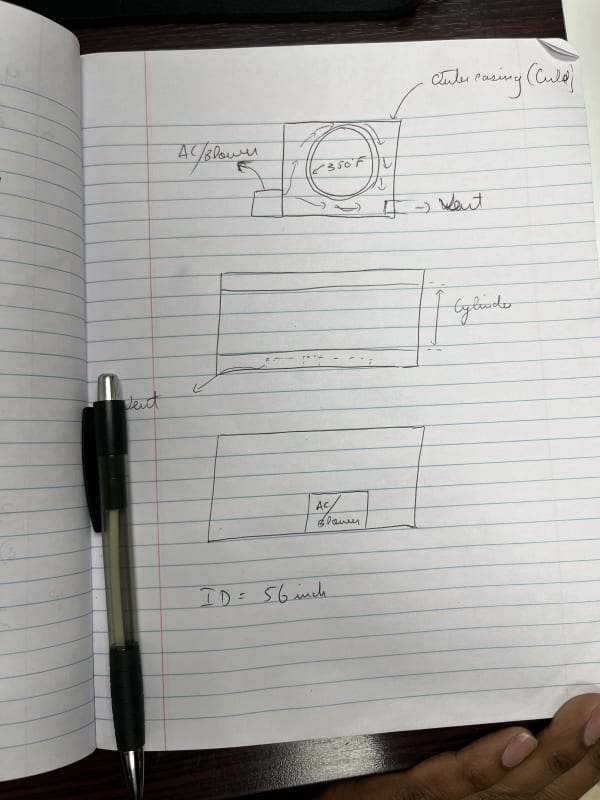
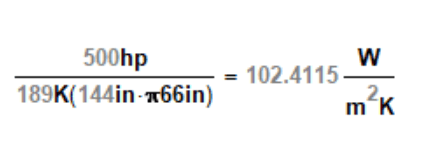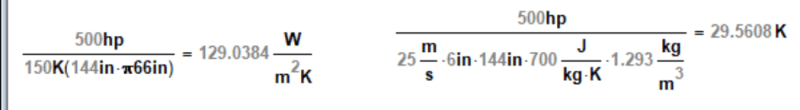OK, then what I calculated is basically the approach.
> you use 500 hp to determine the heat transfer coefficient required of the cooling configuration, and you might need to adjust the surface area downward to account for the flow inefficiency
> you then use the thermal mass capacity of the airflow to determine the linear velocity or volumetric flow of the air and iterate the assumptions as needed, including the boundary layer depth.
So, note that my last calculation wound up with a 56 mph linear velocity, which is pretty crazy; note also the volumetric flow of about 30,000 cfm, which is likewise crazy. These suggest that you need a MASSIVE cooler with an outlet temperature below 0 degC, like say -30 degC. However, since that's below freezing, there will be a crap-ton of condensation to deal with, which does suck cooling capacity from the air stream.
Note also, it's typical that if you want higher transfer efficiency, you'd consider massively increasing the surface area for the heat transfer or consider liquid cooling, which would be 100 times easier vis-a-vis heat transfer, although, obviously, plumbing and pumps are another matter altogether. Note that this latter case simply prolongs the agony, to some degree, since the heat must then be removed from the liquid, but you would have fewer limitations on surface area, and you could use some evaporative transfer at that point.
TTFN (ta ta for now)
I can do absolutely anything. I'm an expert!
faq731-376 forum1529 Entire Forum list