Nerdy123456
Mechanical
- Sep 19, 2017
- 2
The only data in the question is this table:
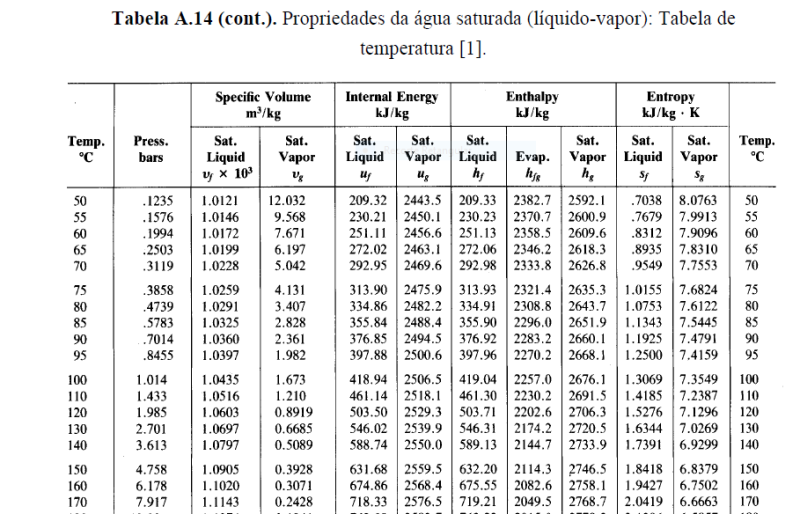
Do we have to do an interpolation between the point at 100ºc + 1.014 bar, the point at 110ºC+ 1.433 bar, and the point at 120ºC + 1.985 bar ?
What other suggestions you may have?
Thanks a lot!![[2thumbsup] [2thumbsup] [2thumbsup]](/data/assets/smilies/2thumbsup.gif)
![[bigears] [bigears] [bigears]](/data/assets/smilies/bigears.gif)

Do we have to do an interpolation between the point at 100ºc + 1.014 bar, the point at 110ºC+ 1.433 bar, and the point at 120ºC + 1.985 bar ?
What other suggestions you may have?
Thanks a lot!
![[2thumbsup] [2thumbsup] [2thumbsup]](/data/assets/smilies/2thumbsup.gif)
![[bigears] [bigears] [bigears]](/data/assets/smilies/bigears.gif)

![[tiphat] [tiphat] [tiphat]](/data/assets/smilies/tiphat.gif)